Question: Setting up differential equation: 1. Middleman 3.38. Write differential equation for species A, assuming its Henry's law constant between gas and liquid is H, the
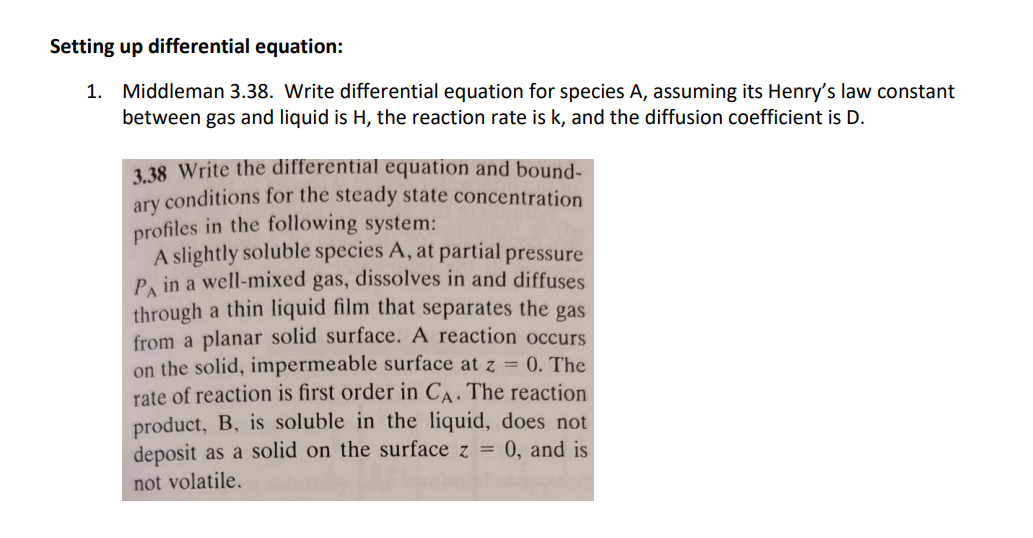
Setting up differential equation: 1. Middleman 3.38. Write differential equation for species A, assuming its Henry's law constant between gas and liquid is H, the reaction rate is k, and the diffusion coefficient is D. 3.38 Write the differential equation and bound- conditions for the steady state concentration profiles in the following system: ary A slightly soluble species A, at partial pressure PA in a well-mixed gas, dissolves in and diffuses through a thin liquid film that separates the gas from a planar solid surface. A reaction occurs on the solid, impermeable surface at z = 0. The rate of reaction is first order in CA. The reaction product, B, is soluble in the liquid, does not deposit as a solid on the surface z = 0, and is not volatile. Setting up differential equation: 1. Middleman 3.38. Write differential equation for species A, assuming its Henry's law constant between gas and liquid is H, the reaction rate is k, and the diffusion coefficient is D. 3.38 Write the differential equation and bound- conditions for the steady state concentration profiles in the following system: ary A slightly soluble species A, at partial pressure PA in a well-mixed gas, dissolves in and diffuses through a thin liquid film that separates the gas from a planar solid surface. A reaction occurs on the solid, impermeable surface at z = 0. The rate of reaction is first order in CA. The reaction product, B, is soluble in the liquid, does not deposit as a solid on the surface z = 0, and is not volatile
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


