Question: SHE are available, such as the silver chloride reference electrode, defined by the following reactions in 3M KCI. Half reactions: Ag(s) SAg (aq) +e AgCl
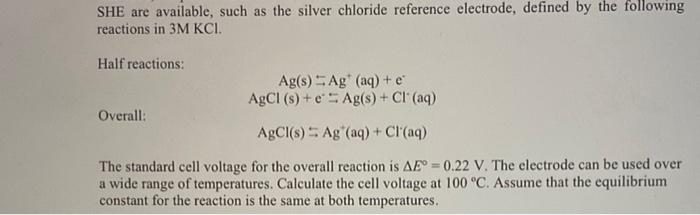
SHE are available, such as the silver chloride reference electrode, defined by the following reactions in 3M KCI. Half reactions: Ag(s) SAg (aq) +e AgCl (8) + e - Ag(s) + Cl(aq) AgCl(s) 5 Ag" (aq) + Cl(aq) Overall: The standard cell voltage for the overall reaction is AE = 0.22 V. The electrode can be used over a wide range of temperatures. Calculate the cell voltage at 100 C. Assume that the equilibrium constant for the reaction is the same at both temperatures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


