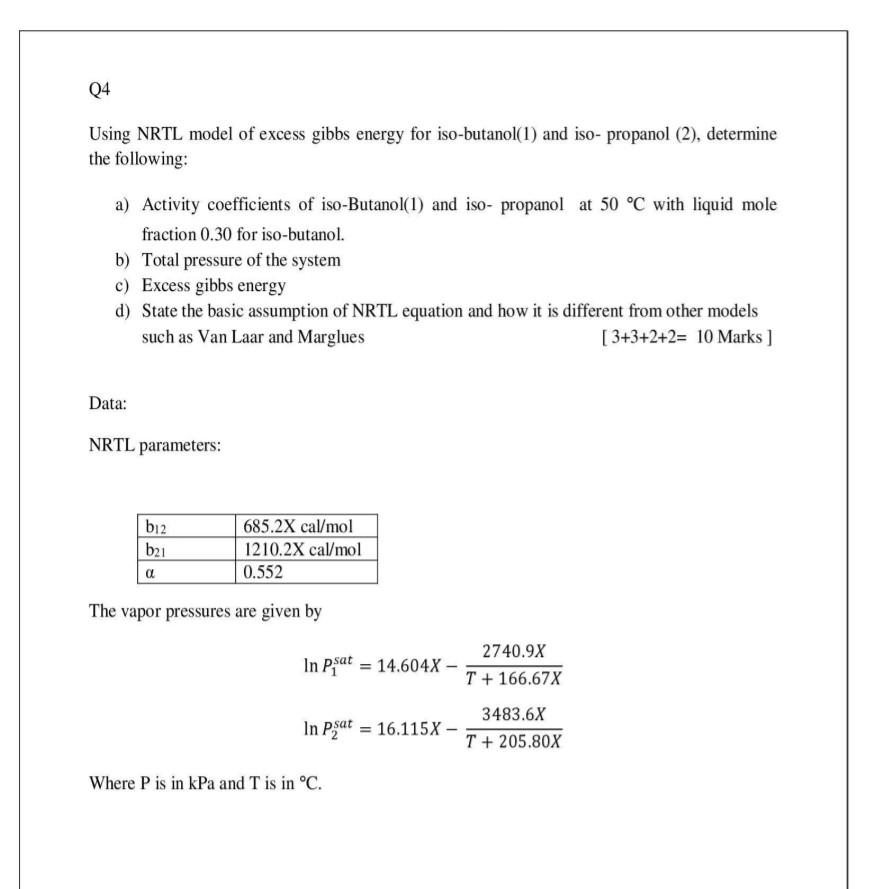
Question: Show all the solution steps please Using NRTL model of excess gibbs energy for iso-butanol(1) and iso- propanol (2), determine the following: a) Activity coefficients
Show all the solution steps please
Using NRTL model of excess gibbs energy for iso-butanol(1) and iso- propanol (2), determine the following: a) Activity coefficients of iso-Butanol(1) and iso- propanol at 50C with liquid mole fraction 0.30 for iso-butanol. b) Total pressure of the system c) Excess gibbs energy d) State the basic assumption of NRTL equation and how it is different from other models such as Van Laar and Marglues [3+3+2+2=10Marks] Data: NRTL parameters: The vapor pressures are given by lnP1sat=14.604XT+166.67X2740.9XlnP2sat=16.115XT+205.80X3483.6X Where P is in kPa and T is in C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


