Question: x=8 Please show all steps of the solution A binary system with Component 1 and Component 2 vapor pressure data are given as function of
x=8
Please show all steps of the solution
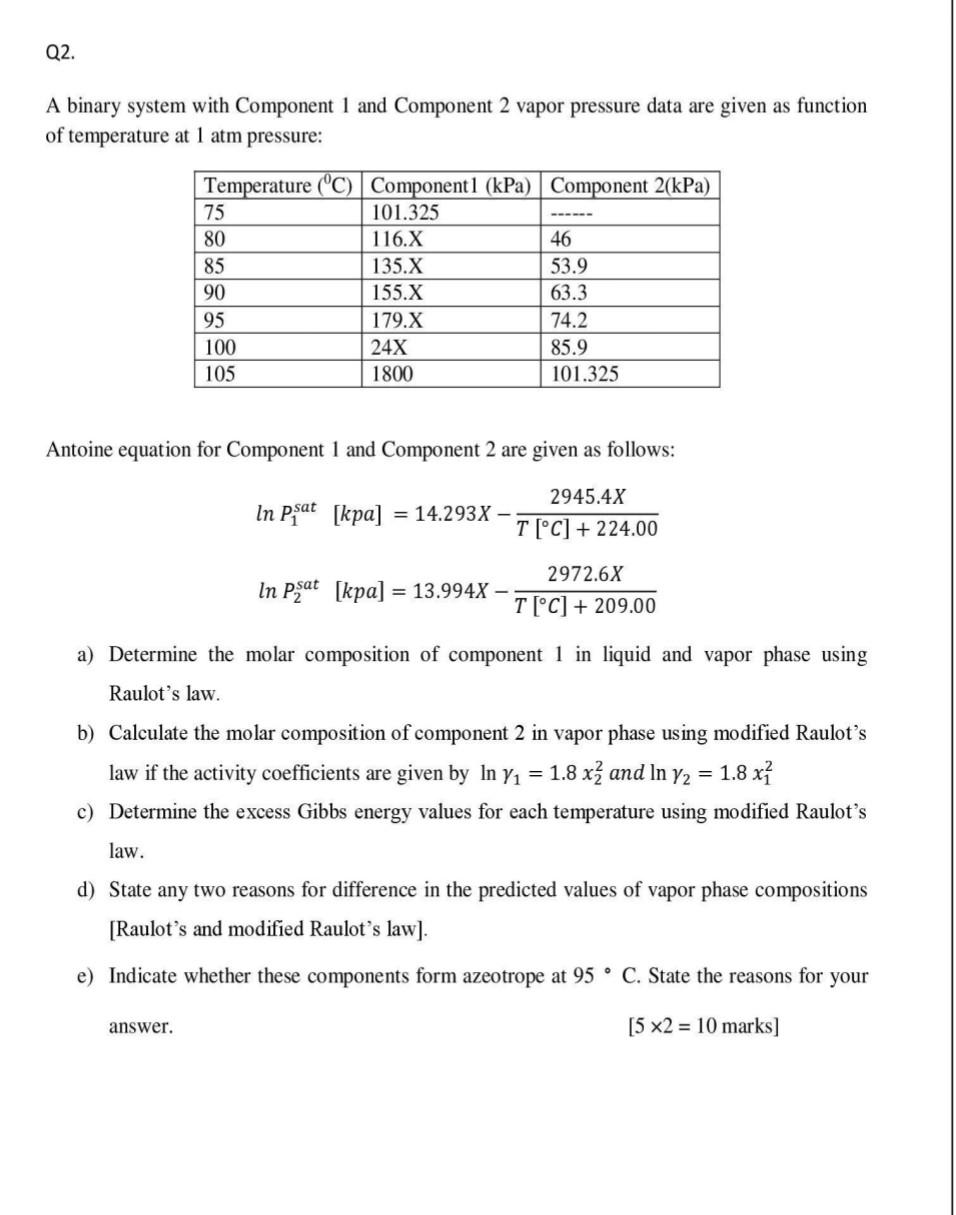
A binary system with Component 1 and Component 2 vapor pressure data are given as function of temperature at 1atm pressure: Antoine equation for Component 1 and Component 2 are given as follows: lnP1sat[kpa]=14.293XT[C]+224.002945.4XlnP2sat[kpa]=13.994XT[C]+209.002972.6X a) Determine the molar composition of component 1 in liquid and vapor phase using Raulot's law. b) Calculate the molar composition of component 2 in vapor phase using modified Raulot's law if the activity coefficients are given by ln1=1.8x22 and ln2=1.8x12 c) Determine the excess Gibbs energy values for each temperature using modified Raulot's law. d) State any two reasons for difference in the predicted values of vapor phase compositions [Raulot's and modified Raulot's law]. e) Indicate whether these components form azeotrope at 95C. State the reasons for your answer. [52=10marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


