Question: show all the steps please Q3: Ammonia (A) diffuses through a stagnant layer of air (B), 1.0 cm thickness, at 25C and 1.0 atm total
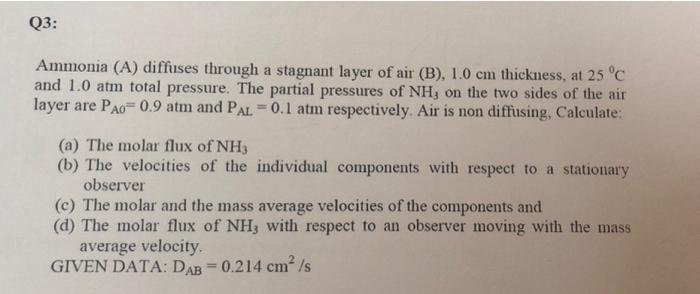

Q3: Ammonia (A) diffuses through a stagnant layer of air (B), 1.0 cm thickness, at 25C and 1.0 atm total pressure. The partial pressures of NH, on the two sides of the air layer are Pao= 0.9 atm and PAL = 0.1 atm respectively. Air is non diffusing, Calculate: (a) The molar flux of NH3 (b) The velocities of the individual components with respect to a stationary observer (c) The molar and the mass average velocities of the components and (d) The molar flux of NH3 with respect to an observer moving with the mass average velocity GIVEN DATA: DAB=0.214 cm/s Q1: Mass analysis of a gas mixture at 290 K and 250 kPa is 35g, N2, 20g O2 and 10g CO2. Determine the mass & mole fractions and partial pressure of each gas
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


