Question: show and explain the complete steps to answer this question. (20 marks) Hydrogen is produced in the steam reforming of propane: C3H8 (g) + 3H20
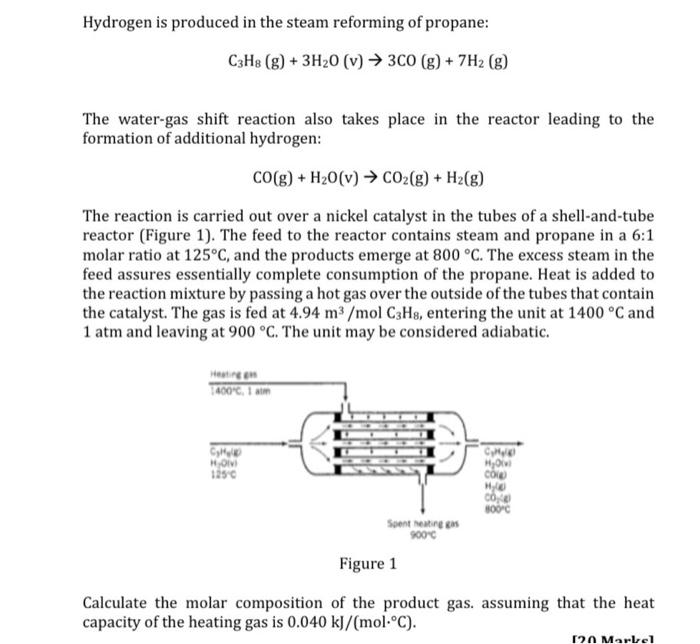
Hydrogen is produced in the steam reforming of propane: C3H8 (g) + 3H20 (v) 3C0 (g) + 7H2 (g) The water-gas shift reaction also takes place in the reactor leading to the formation of additional hydrogen: CO(g) + H20(v) CO2(g) + H2(g) The reaction is carried out over a nickel catalyst in the tubes of a shell-and-tube reactor (Figure 1). The feed to the reactor contains steam and propane in a 6:1 molar ratio at 125C, and the products emerge at 800 C. The excess steam in the feed assures essentially complete consumption of the propane. Heat is added to the reaction mixture by passing a hot gas over the outside of the tubes that contain the catalyst. The gas is fed at 4.94 m3/mol C3Hs, entering the unit at 1400 C and 1 atm and leaving at 900 C. The unit may be considered adiabatic. Het en 400C, 1 am CHU HO 1250 HO CON MO COX 300C Sontreatineens 900c Figure 1 Calculate the molar composition of the product gas, assuming that the heat capacity of the heating gas is 0.040 kJ/(mol.C). 120 Marel
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


