Question: show material balance HW : Group Activity Process Flow Chart Nitric acid is used extensively for the production of inorganic and organic nitrates, for metal
show material balance 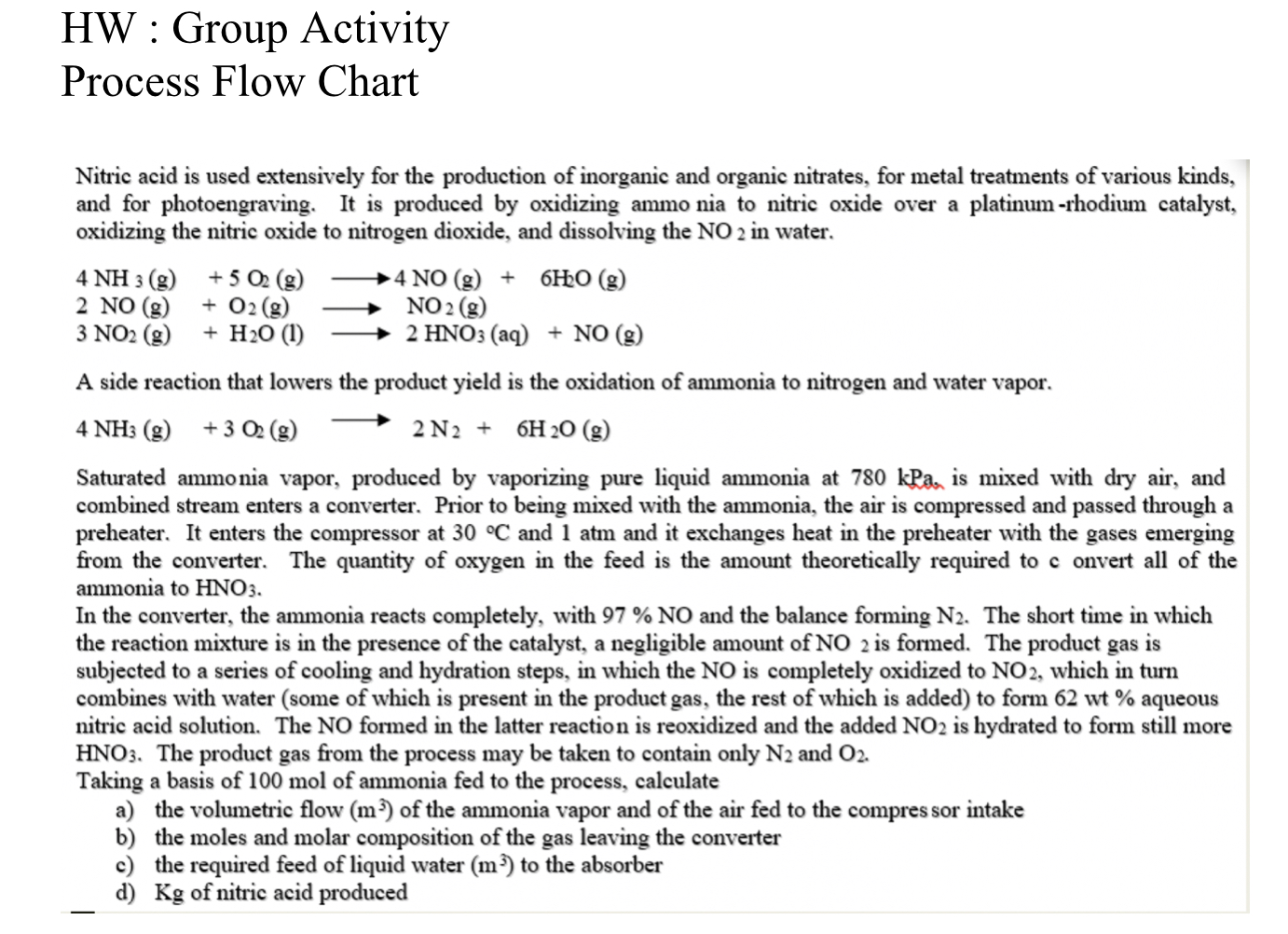
HW : Group Activity Process Flow Chart Nitric acid is used extensively for the production of inorganic and organic nitrates, for metal treatments of various kinds, and for photoengraving. It is produced by oxidizing ammo nia to nitric oxide over a platinum -rhodium catalyst, oxidizing the nitric oxide to nitrogen dioxide, and dissolving the NO2 in water. 4NH3(g)+5O2(g)4NO(g)+6HO(g)2NO(g)+O2(g)NO2(g)3NO2(g)+H2O(l)2HNO3(aq)+NO(g) A side reaction that lowers the product yield is the oxidation of ammonia to nitrogen and water vapor. 4NH3(g)+3O2(g)2N2+6H2O(g) Saturated ammonia vapor, produced by vaporizing pure liquid ammonia at 780kPa, is mixed with dry air, and combined stream enters a converter. Prior to being mixed with the ammonia, the air is compressed and passed through a preheater. It enters the compressor at 30C and 1atm and it exchanges heat in the preheater with the gases emerging from the converter. The quantity of oxygen in the feed is the amount theoretically required to c onvert all of the ammonia to HNO3. In the converter, the ammonia reacts completely, with 97%NO and the balance forming N2. The short time in which the reaction mixture is in the presence of the catalyst, a negligible amount of NO2 is formed. The product gas is subjected to a series of cooling and hydration steps, in which the NO is completely oxidized to NO2, which in turn combines with water (some of which is present in the product gas, the rest of which is added) to form 62 wt % aqueous nitric acid solution. The NO formed in the latter reaction is reoxidized and the added NO2 is hydrated to form still more HNO3. The product gas from the process may be taken to contain only N2 and O2. Taking a basis of 100mol of ammonia fed to the process, calculate a) the volumetric flow (m3) of the ammonia vapor and of the air fed to the compres sor intake b) the moles and molar composition of the gas leaving the converter c) the required feed of liquid water (m3) to the absorber d) Kg of nitric acid produced
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


