Question: Show me how they got the correct answer please. The equilibrium bond length in nitrogen gas (14N2) is 1.10A. (Use the relative atomic mass: 14N=14.003074005.)
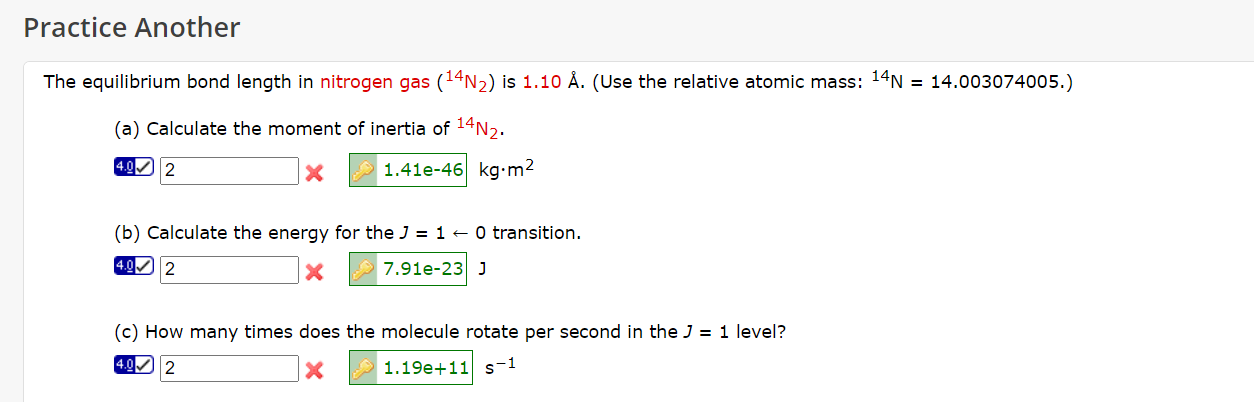
Show me how they got the correct answer please.
The equilibrium bond length in nitrogen gas (14N2) is 1.10A. (Use the relative atomic mass: 14N=14.003074005.) (a) Calculate the moment of inertia of 14N2. 4.00 *kgm2 (b) Calculate the energy for the J=10 transition. 4.0 2 J (c) How many times does the molecule rotate per second in the J=1 level? * s1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


