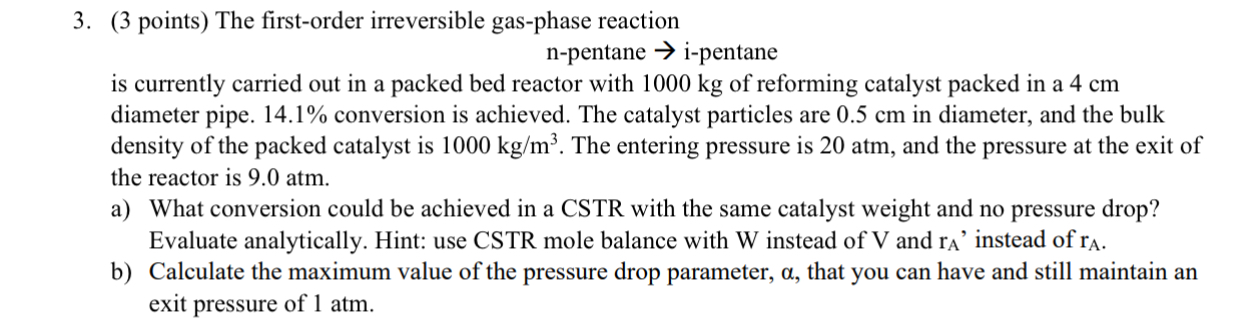
Question: SHOW POLYMATH CODE!! The first - order irreversible gas - phase reaction n - pentane i - pentane is currently carried out in a packed
SHOW POLYMATH CODE!!
The firstorder irreversible gasphase reaction
npentane ipentane is currently carried out in a packed bed reactor with of reforming catalyst packed in a diameter pipe. conversion is achieved. The catalyst particles are in diameter, and the bulk density of the packed catalyst is The entering pressure is atm, and the pressure at the exit of the reactor is atm.
a What conversion could be achieved in a CSTR with the same catalyst weight and no pressure drop? Evaluate analytically. Hint: use CSTR mole balance with instead of and instead of
b Calculate the maximum value of the pressure drop parameter, that you can have and still maintain an exit pressure of atm.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


