Question: show work for 2-5 please 1. Draw the most stable chair conformation of cis1-fluoro-4-isopropylcyclohexane. 2. Consider this compound: a) How many chiral carbons does this
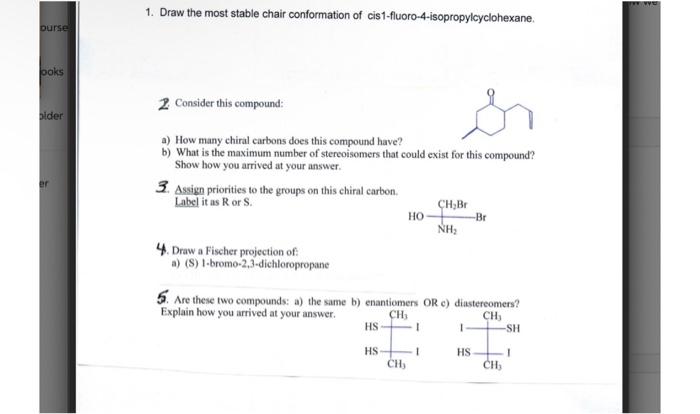


1. Draw the most stable chair conformation of cis1-fluoro-4-isopropylcyclohexane. 2. Consider this compound: a) How many chiral carbons does this compound have? b) What is the maximum number of stereoisomers that could exist for this compound? Show how you arrived at your answer. 3. Assign priorities to the groups on this chiral carbon. Label it as R or S. 4. Draw a Fischer projection of: a) (S) 1-bromo-2,3-dichloropropane 5. Are these two compounds: a) the same bi enantiomers OR al dinstamonmase? Explain how you arrived at your answer. organic chemistry 1 1. Draw the most stable chair conformation of cis1-fluoro-4-isopropylcyclohexane. 2. Consider this compound: a) How many chiral carhons does this compound have? b) What is the maximum number of stereoisomers that could exist for this compound? Show how you arrived at your answer. 3. Assign priorities to the groups on this chiral carbon. Label it as R or S. 4. Draw a Fischer projection of: a) (S) 1-bromo-2,3-dichloropropane 5. Are these two compounds: a) the same b) enantiomers OR c) diastercomers? Explain how you arrived at your answer. 1. Draw the most stable chair conformation of cis1-fluoro-4-isopropylcyclohexane. 2. Consider this compound: a) How many chiral carbons does this compound have? b) What is the maximum number of stereoisoeners that could exist for this compound? Show how you arrived at your answer. 3. Assiga priorities to the groupe on this chiral carbon. label it as R or S. Draw a Fischer projection of: a) (S) 1-bromo-2,3-dichloropropane 5. Are these two compounds: a) the same b) enantiomers OR cl diastereneners? Explain bow you arrived at your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


