Question: show work for 4 and 5 please 4) Consider the container below. The volume of the left side and the volume of the right side
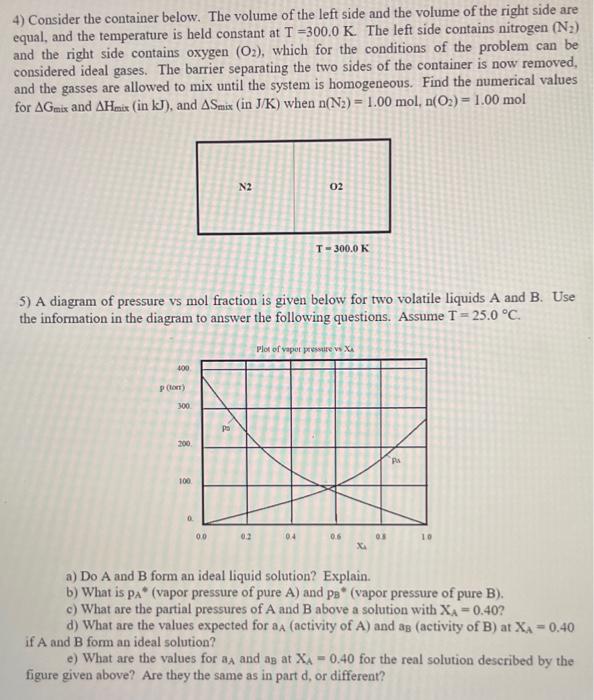
4) Consider the container below. The volume of the left side and the volume of the right side are equal, and the temperature is held constant at T=300.0K. The left side contains nitrogen (N2) and the right side contains oxygen (O2), which for the conditions of the problem can be considered ideal gases. The barrier separating the two sides of the container is now removed, and the gasses are allowed to mix until the system is homogeneous. Find the numerical values for Gmix and Hmix (in kJ), and Smix (in J/K ) when n(N2)=1.00mol,n(O2)=1.00mol 5) A diagram of pressure vs mol fraction is given below for two volatile liquids A and B. Use the information in the diagram to answer the following questions. Assume T=25.0C. a) Do A and B form an ideal liquid solution? Explain. b) What is pAA " (vapor pressure of pure A ) and pB (vapor pressure of pure B). c) What are the partial pressures of A and B above a solution with XA=0.40 ? d) What are the values expected for aA (activity of A ) and aB (activity of B ) at XA=0.40 if A and B form an ideal solution? e) What are the values for aA and aB at XA=0.40 for the real solution described by the figure given above? Are they the same as in part d, or different
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


