Question: Show work please. DATA ANALYSIS 1.Calculate molality (m), in mol/ kg, using the formula =Kfm. The Kf value for lauric acid is 3.9Ckg/mol. 2.Calculate moles
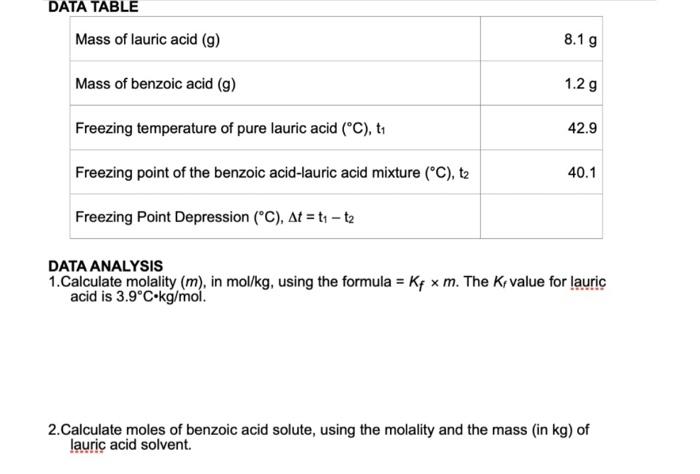
DATA ANALYSIS 1.Calculate molality (m), in mol/ kg, using the formula =Kfm. The Kf value for lauric acid is 3.9Ckg/mol. 2.Calculate moles of benzoic acid solute, using the molality and the mass (in kg ) of lauric acid solvent
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


