Question: show work please Problem 3 (40 points) Heat Loss During Breathing We will estimate the rate of heat loss due to the moist air expired
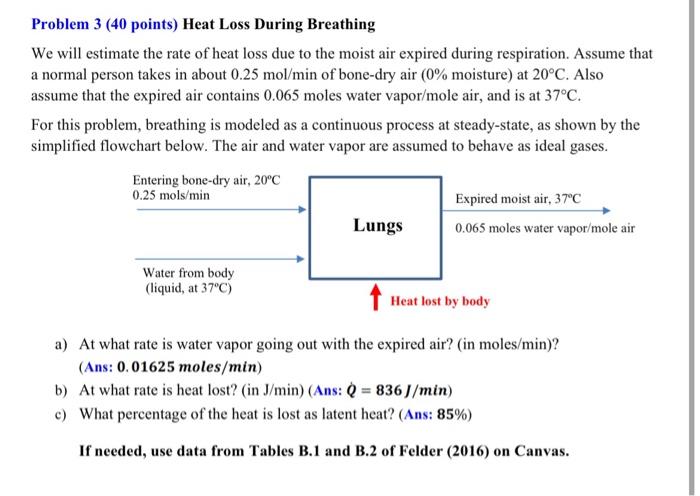
Problem 3 (40 points) Heat Loss During Breathing We will estimate the rate of heat loss due to the moist air expired during respiration. Assume that a normal person takes in about 0.25 mol/min of bone-dry air (0% moisture) at 20C. Also assume that the expired air contains 0.065 moles water vapor/mole air, and is at 37C. For this problem, breathing is modeled as a continuous process at steady-state, as shown by the simplified flowchart below. The air and water vapor are assumed to behave as ideal gases. Entering bone-dry air, 20C 0.25 mols/min Expired moist air, 37C Lungs 0.065 moles water vapor/mole air Water from body (liquid, at 37C) Heat lost by body a) At what rate is water vapor going out with the expired air? (in moles/min)? (Ans: 0.01625 moles/min) b) At what rate is heat lost? (in J/min) (Ans: Q = 836J/min) c) What percentage of the heat is lost as latent heat? (Ans: 85%) If needed, use data from Tables B.1 and B.2 of Felder (2016) on Canvas. Problem 3 (40 points) Heat Loss During Breathing We will estimate the rate of heat loss due to the moist air expired during respiration. Assume that a normal person takes in about 0.25 mol/min of bone-dry air (0% moisture) at 20C. Also assume that the expired air contains 0.065 moles water vapor/mole air, and is at 37C. For this problem, breathing is modeled as a continuous process at steady-state, as shown by the simplified flowchart below. The air and water vapor are assumed to behave as ideal gases. Entering bone-dry air, 20C 0.25 mols/min Expired moist air, 37C Lungs 0.065 moles water vapor/mole air Water from body (liquid, at 37C) Heat lost by body a) At what rate is water vapor going out with the expired air? (in moles/min)? (Ans: 0.01625 moles/min) b) At what rate is heat lost? (in J/min) (Ans: Q = 836J/min) c) What percentage of the heat is lost as latent heat? (Ans: 85%) If needed, use data from Tables B.1 and B.2 of Felder (2016) on Canvas
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


