Question: Shown below are the absorption (A) and emission (8) spectra for lithium atoms (Note in the absorption spectrum, the black lines represent wavelengths that were
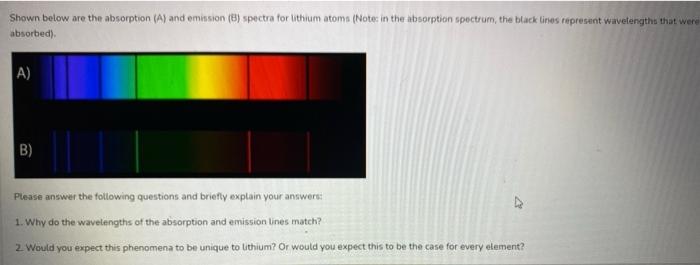
Shown below are the absorption (A) and emission (8) spectra for lithium atoms (Note in the absorption spectrum, the black lines represent wavelengths that were absorbed) A) B) B) Please answer the following questions and briefly explain your answers: 1. Why do the wavelengths of the absorption and emission lines match? 2 Would you expect this phenomena to be unique to lithium? Or would you expect this to be the case for every element
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


