Question: Shown below is data from a titration experiment that was performed using HCI, CH3COOH and C H2O7 and H2SO4 of unknown concentration. Different dilutions of
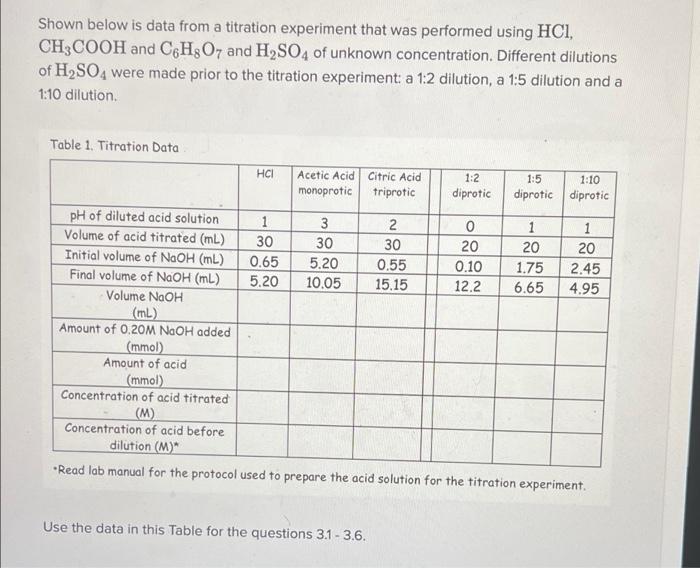

Shown below is data from a titration experiment that was performed using HCI, CH3COOH and C H2O7 and H2SO4 of unknown concentration. Different dilutions of H2SO4 were made prior to the titration experiment a 1:2 dilution, a 1:5 dilution and a 1:10 dilution. Table 1. Titration Data HCI Acetic Acid citric Acid monoprotic triprotic 1:2 diprotic 1:5 1:10 diprotic diprotic pH of diluted acid solution 1 3 2 0 1 1 Volume of acid titrated (mL) 30 30 30 20 20 20 Initial volume of NaOH (mL) 0.65 5.20 0.55 0.10 1.75 2.45 Final volume of NaOH (mL) 5.20 10.05 15.15 12.2 6.65 4.95 Volume NaOH (ml) Amount of 0.20M NaOH added (mmol) Amount of acid (mmol) Concentration of acid titrated (M) Concentration of acid before dilution (M)* Read lab manual for the protocol used to prepare the acid solution for the titration experiment. Use the data in this Table for the questions 3.1 - 3.6. Q3.2 1.25 Points Determine the following using the data obtained for the acetic acid (a monoprotic acid) titration experiment (note Table above). Please make certain to show your work and that your final answers are well marked. 1. volume of NaOH 2. the mmoles of 0.2M NaOH added 3. the mmoles of acid 4. the concentration of the acetic acid titrated 5. the concentration of the acetic acid before dilution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


