Question: ..slations pH = Weak Base Calculations Sodium Phosphate Conjugate Calculate the pH of 0.10 M NPO solution Acid Hydrolysis Base Reaction ka 2.4E-02 Kb E
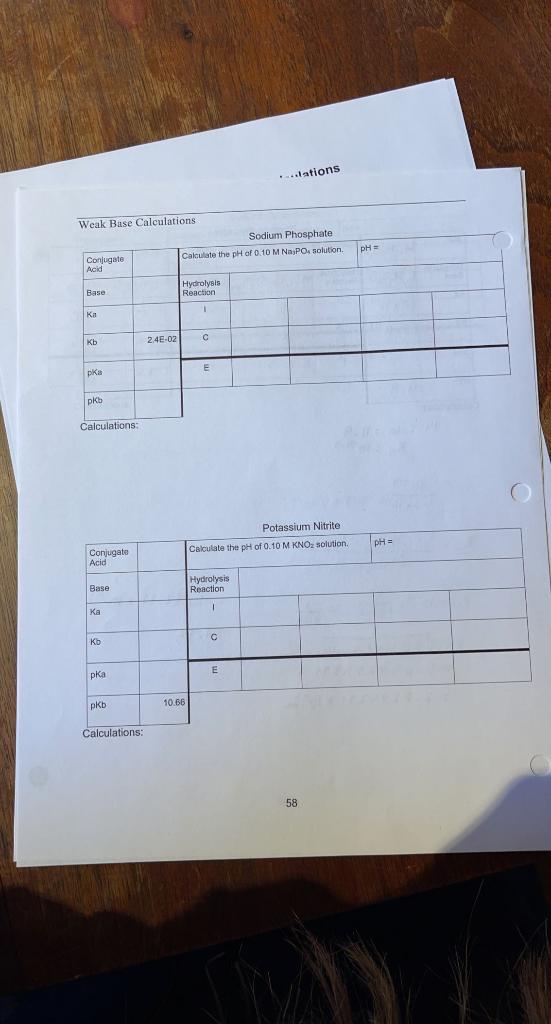
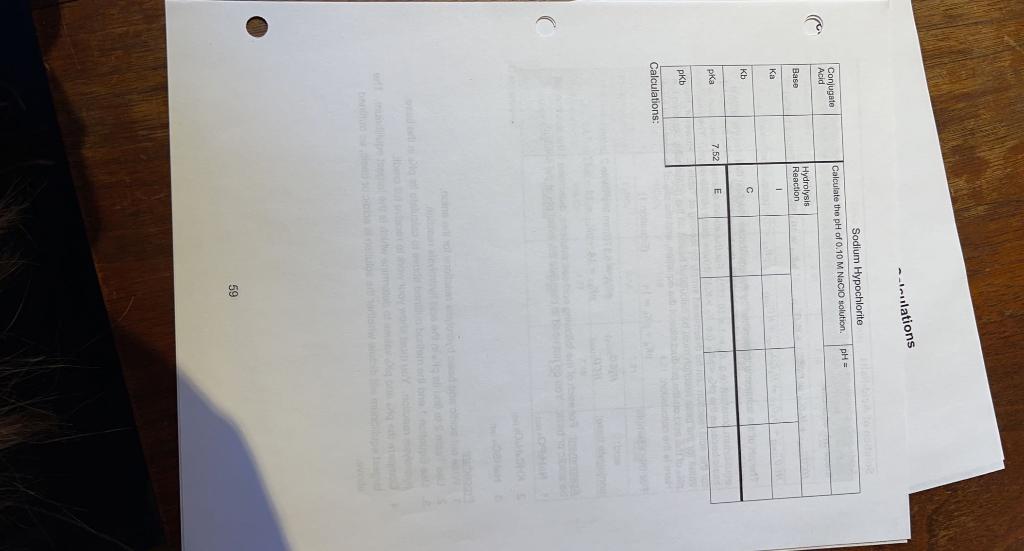
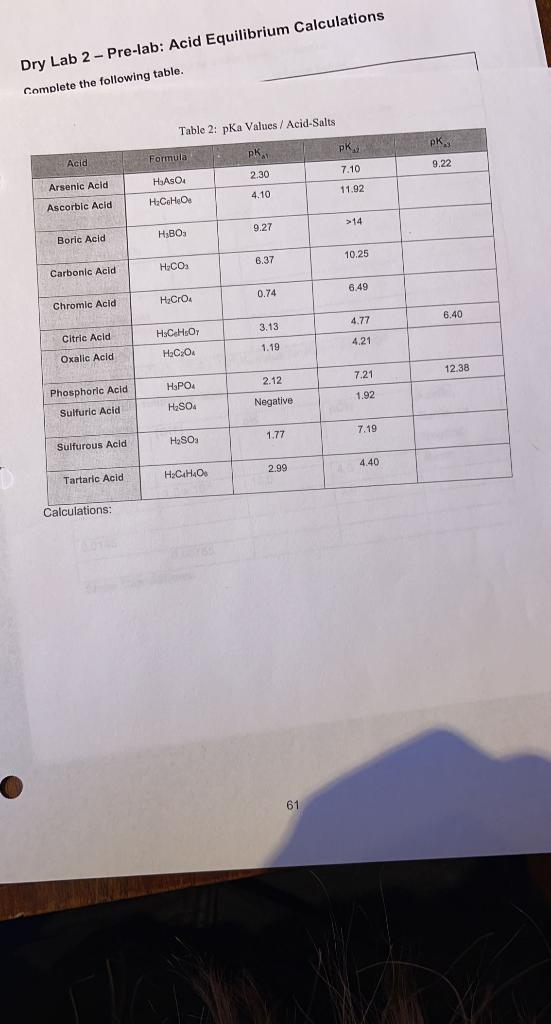
..slations pH = Weak Base Calculations Sodium Phosphate Conjugate Calculate the pH of 0.10 M NPO solution Acid Hydrolysis Base Reaction ka 2.4E-02 Kb E pka pKb Calculations Potassium Nitrite Calculate the pH of 0.10 M KNOZ solution. pH = Conjugate Acid Base Hydrolysis Reaction 1 Ka Kb E pka pkb 10.66 Calculations: 58 Innlations Sodium Hypochlorite Calculate the pH of 0.10 M Nacio solution pH = Conjugate Acid Baso Hydrolysis Reaction | Kb E 7.52 Calculations: 59 Dry Lab 2 - Pre-lab: Acid Equilibrium Calculations Complete the following table. Table 2: pKa Values / Acid-Salts pK pk. pk. Acid Formula 9.22 2.30 7.10 Arsenic Acid HASO 11.92 4.10 Ascorbic Acid > 14 9.27 Boric Acid HBO 10.25 6.37 H.CO Carbonic Acid 6.49 0.74 Chromic Acid H.Cro 6.40 4.77 3.13 Citric Acid H.C.H.Oy 4.21 1.19 Oxalic Acid Hec 04 12.38 7.21 2.12 HPO4 1.92 Phosphoric Acid Sulfuric Acid H2SO4 Negative 1.77 7.19 H2SO3 Sulfurous Acid 4.40 2.99 H.C.H.O. Tartaric Acid Calculations: 61
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


