Question: SMITH CHEMICAL ENGINEERING KINETICS 1 2 - 3 The liquid - phase hydrogenation of - methyl styrene to cumene, H 2 ( dissolved ) +
SMITH CHEMICAL ENGINEERING KINETICS
The liquidphase hydrogenation of methyl styrene to cumene,
dissolved
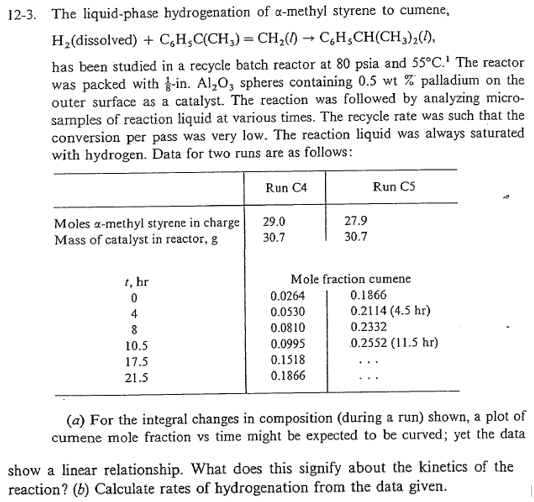
has been studied in a recycle batch reactor at psia and The reactor was packed with in spheres containing palladium on the outer surface as a catalyst. The reaction was followed by analyzing microsamples of reaction liquid at various times. The recycle rate was such that the conversion per pass was very low. The reaction liquid was always saturated
with hydrogen. Data for two runs are as follows:
table
a For the integral changes in composition during a run shown, a plot of cumene mole fraction vs time might be expected to be curved; yet the data show a linear relationship. What does this signify about the kinetics of the reaction?
b Calculate rates of hydrogenation from the data given.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


