Question: Solid silicon in contact with solid silicon dioxide is to be heated to a temperature of 1100 K in a vacuum furnace. The two solid
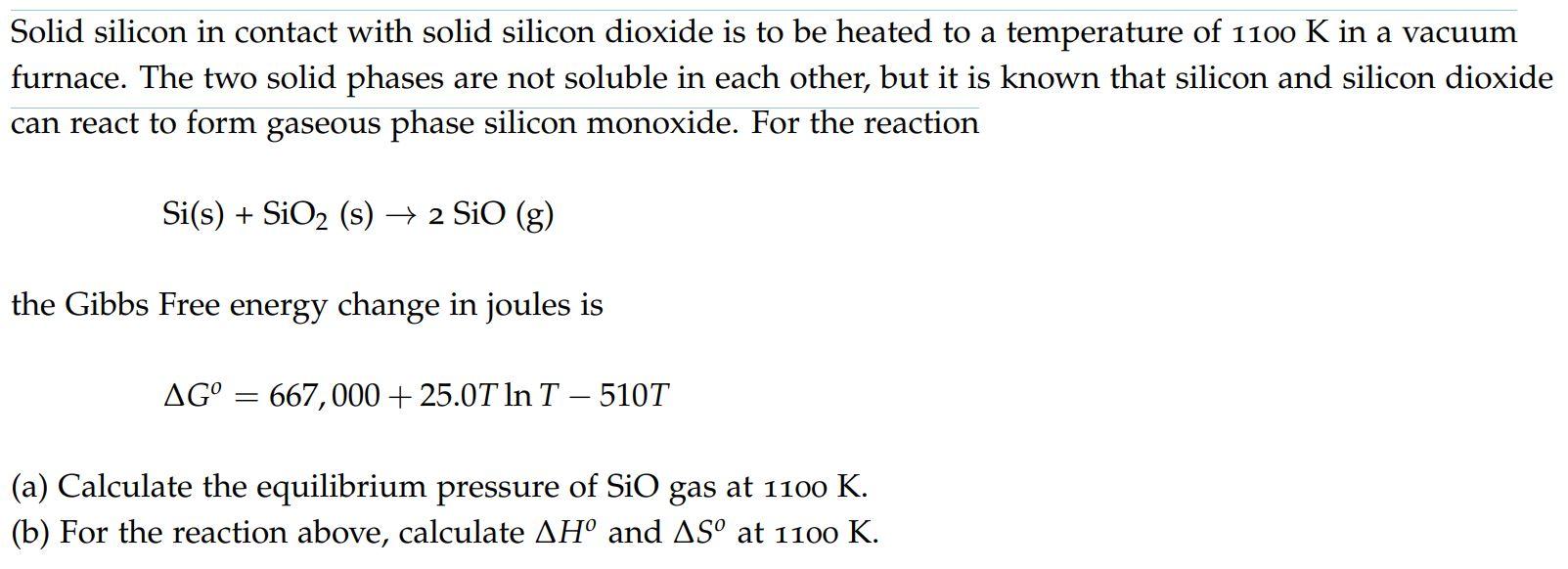
Solid silicon in contact with solid silicon dioxide is to be heated to a temperature of 1100 K in a vacuum furnace. The two solid phases are not soluble in each other, but it is known that silicon and silicon dioxide can react to form gaseous phase silicon monoxide. For the reaction Si(s) + SiO2 (s) + 2 SiO (g) the Gibbs Free energy change in joules is AG = 667,000 + 25.0T In T - 510T (a) Calculate the equilibrium pressure of SiO gas at 1100 K. (b) For the reaction above, calculate H and AS at 1100 K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


