Question: Solid sulfur undergoes a phase transition between the monoclinic (m) and orthorhombic (o) phases at a temperature of 368.3 K and pressure of 1
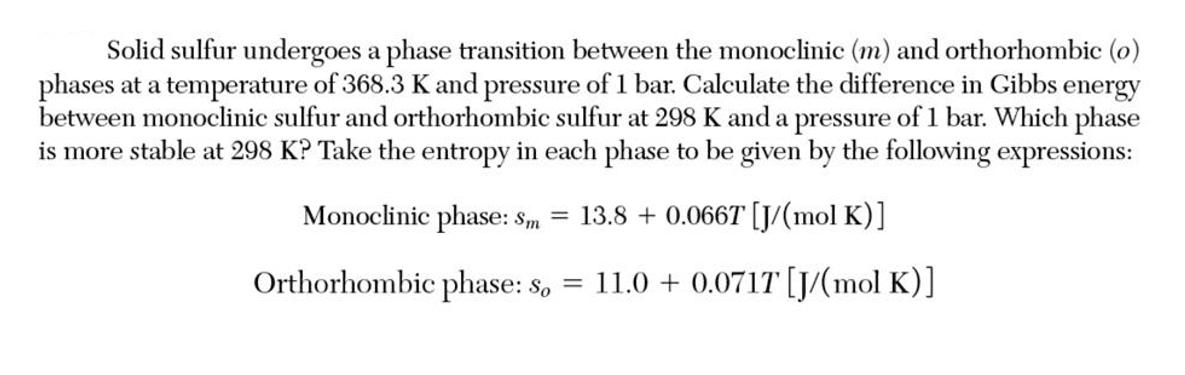
Solid sulfur undergoes a phase transition between the monoclinic (m) and orthorhombic (o) phases at a temperature of 368.3 K and pressure of 1 bar. Calculate the difference in Gibbs energy between monoclinic sulfur and orthorhombic sulfur at 298 K and a pressure of 1 bar. Which phase is more stable at 298 K? Take the entropy in each phase to be given by the following expressions: Monoclinic phase: Sm = 13.8 + 0.066T [J/(mol K)] Orthorhombic phase: so = 11.0+ 0.0717 [J/(mol K)]
Step by Step Solution
3.38 Rating (167 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


