Question: Solid Type: Ionic Unit 1: Structure and Properties of Matter: /6 marks Background: We have studied the properties of various solids and have classified them
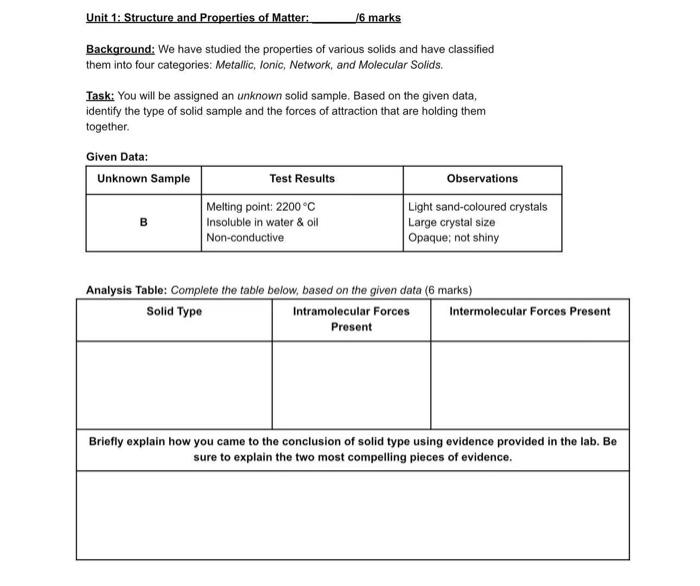
Unit 1: Structure and Properties of Matter: /6 marks Background: We have studied the properties of various solids and have classified them into four categories: Metallic, Ionic, Network, and Molecular Solids. Task: You will be assigned an unknown solid sample. Based on the given data, identify the type of solid sample and the forces of attraction that are holding them together. Given Data: Unknown Sample Test Results Observations Melting point: 2200C Light sand-coloured crystals Insoluble in water & oil Large crystal size Non-conductive Opaque; not shiny B Analysis Table: Complete the table below, based on the given data (6 marks) Solid Type Intramolecular Forces Intermolecular Forces Present Present Briefly explain how you came to the conclusion of solid type using evidence provided in the lab. Be sure to explain the two most compelling pieces of evidence
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


