Question: Solution pH from Indicator pH from Indicator pH from Meter 0.1M HCl 1 1.2-2.8 1.02 0.1M H3PO4 2 1.2-2.8 1.48 0.1M HC2H3O2, 3 1.2-2.8 2.64
| Solution | pH from Indicator | pH from Indicator | pH from Meter |
| 0.1M HCl | 1 | 1.2-2.8 | 1.02 |
| 0.1M H3PO4 | 2 | 1.2-2.8 | 1.48 |
| 0.1M HC2H3O2, | 3 | 1.2-2.8 | 2.64 |
| 0.1M NaH2PO4, | 5 | 4.5-6.0 | 4.49 |
| 0.1M Al(NO3)3, | 3 | 2.8-3.1 | 2.80 |
| 0.1M Zn(NO3)2 | 6 | 4.5-6.0 | 6.03 |
| 0.1M NH4NO3 | 6 | 1.2-2.8 | 5.48 |
 a. Calculate the concentration of H3O+ ions in the least acidic solution that you examined. Use your most precise pH measurement.
a. Calculate the concentration of H3O+ ions in the least acidic solution that you examined. Use your most precise pH measurement.
b. Arrange the acids that you used in order of decreasing acidity. Use your most precise pH measurements. Exclude the common household substances.
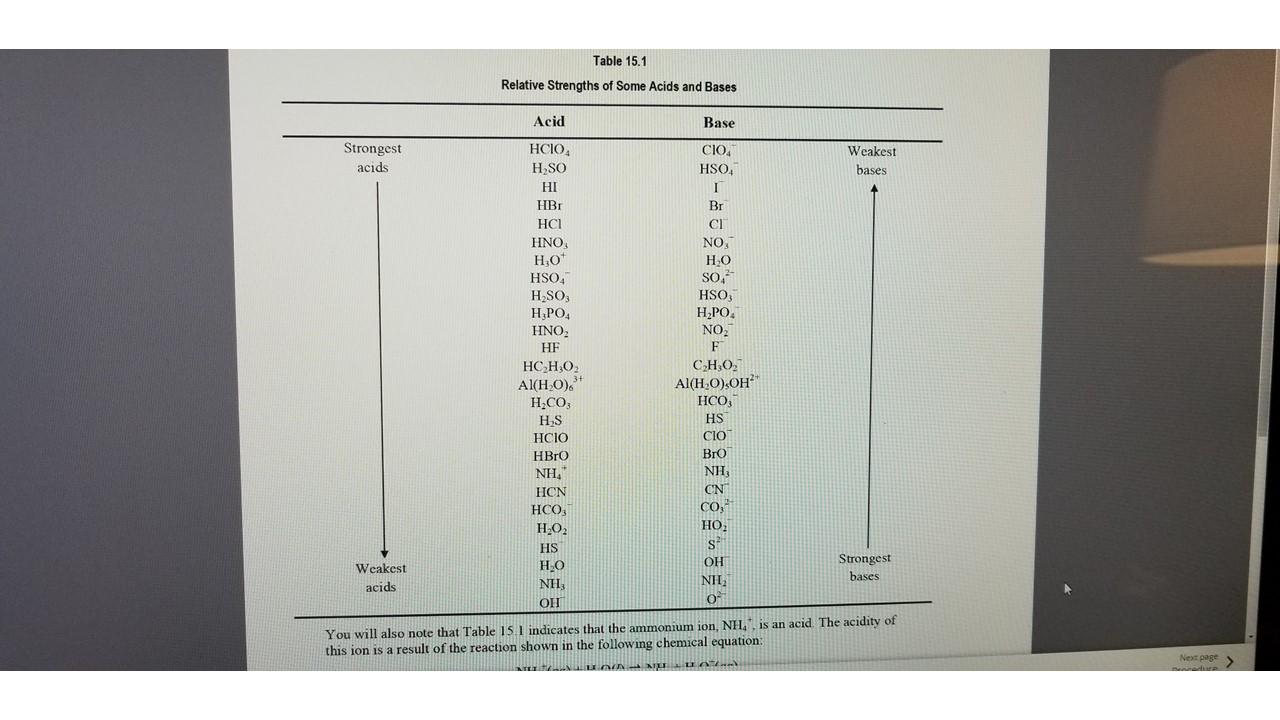
c. How does your arrangement compare with the first column in Table 15.1, insofar as a comparison can be made? Comment on the similarities and differences.
d. Comment on the relative acidities of H3PO4 and the H2PO4+ ion.
e. Write the balanced chemical reactions that show why solutions of Al(NO3)3, Zn(NO3)2, and NH4NO3 have the pH values that you found.
f. Arrange the common household substances in order of decreasing acidity.
Table 15.1 Relative Strengths of Some Acids and Bases Acid Base Strongest acids Weakest bases HCIO , H SO HI HBI HCI , H0+ HSOA H_SO , HNO, HE HCHO 3+ Al(HO). H.CO; HS HCIO HBro NH, HCN HCO3 H2O2 HS HO NH OH CIO HSO. 1 Br CI , , HO SO, HSO3 H2PO. NO. F C,H,O, Al(HO),OH HCO3 HS CIO Bro NH ON CO, , , S OH NH, 04 Weakest acids Strongest bases You will also note that Table 15 | indicates that the ammonium ion, NH, is an acid. The acidity of this ion is a result of the reaction shown in the following chemical equation: RUNNIN Next page Table 15.1 Relative Strengths of Some Acids and Bases Acid Base Strongest acids Weakest bases HCIO , H SO HI HBI HCI , H0+ HSOA H_SO , HNO, HE HCHO 3+ Al(HO). H.CO; HS HCIO HBro NH, HCN HCO3 H2O2 HS HO NH OH CIO HSO. 1 Br CI , , HO SO, HSO3 H2PO. NO. F C,H,O, Al(HO),OH HCO3 HS CIO Bro NH ON CO, , , S OH NH, 04 Weakest acids Strongest bases You will also note that Table 15 | indicates that the ammonium ion, NH, is an acid. The acidity of this ion is a result of the reaction shown in the following chemical equation: RUNNIN Next page
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


