Question: solution The incorrect statement(s) regarding 2M MgCl, aqueous solution is/are (d (a) Molality of Cl is 4.44 m (b) Mole fraction of MgCl is
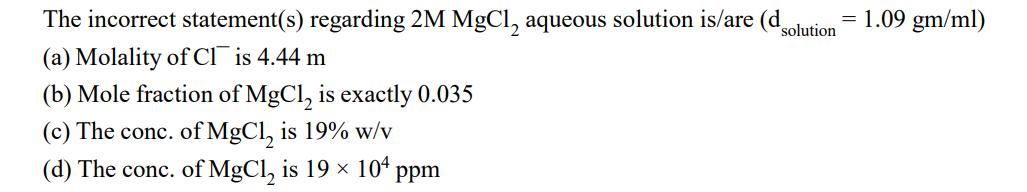
solution The incorrect statement(s) regarding 2M MgCl, aqueous solution is/are (d (a) Molality of Cl is 4.44 m (b) Mole fraction of MgCl is exactly 0.035 (c) The conc. of MgCl, is 19% w/v (d) The conc. of MgCl is 19 104 ppm = 1.09 gm/ml)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


