Question: solution. This solution is mixed thoroughly in a batch process with 3.0kg of granular activated carbon until equilibrium is reached Use the isotherm from Example

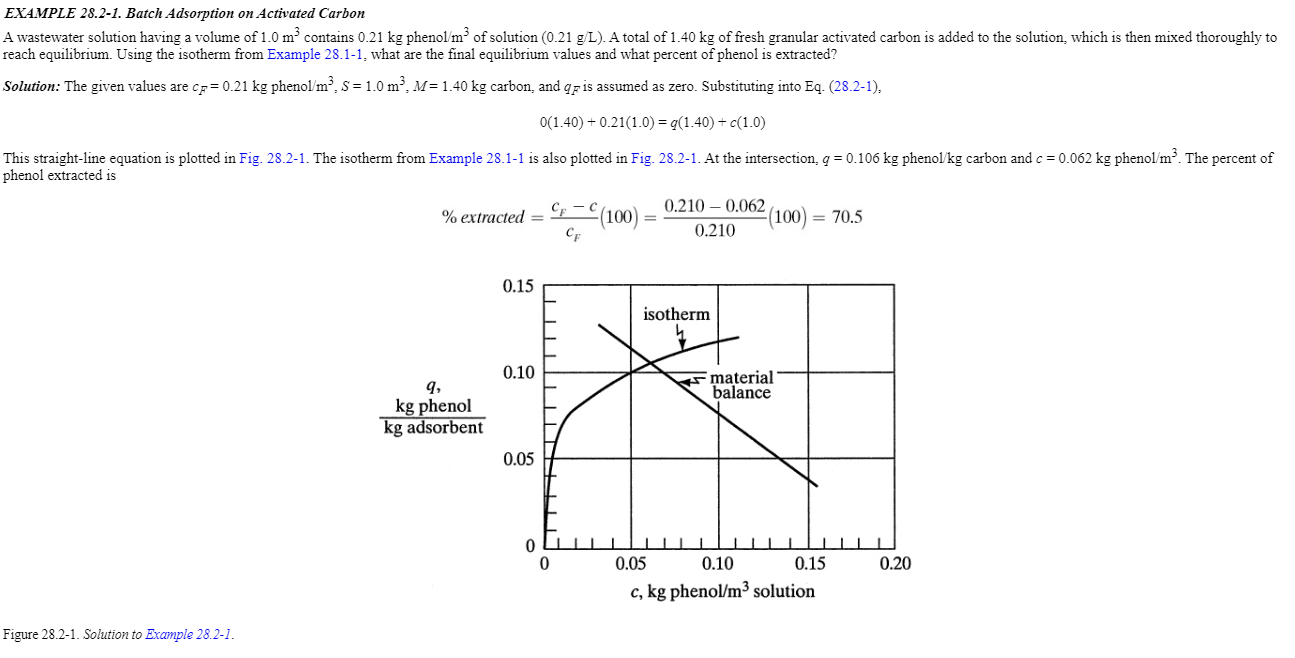
solution. This solution is mixed thoroughly in a batch process with 3.0kg of granular activated carbon until equilibrium is reached Use the isotherm from Example 28.2-1 and calculate the final equilibrium values and the percent phenol extracted. EXAMPLE 28.2-1. Batch Adsorption on Activated Carbon reach equilibrium. Using the isotherm from Example 28.1-1, what are the final equilibrium values and what percent of phenol is extracted? Solution: The given values are cF=0.21kg phenol /m3,S=1.0m3,M=1.40kg carbon, and qF is assumed as zero. Substituting into Eq. (28.2-1), 0(1.40)+0.21(1.0)=q(1.40)+c(1.0) phenol extracted is %extracted=cFcFc(100)=0.2100.2100.062(100)=70.5 Figure 28.2-1. Solution to Example 28.2-1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


