Question: Solve 2. (a-d): This the the third problem: This is problem 6.8: 2. Consider the semibatch reactor problem from the last homework (Problem 6.8 )


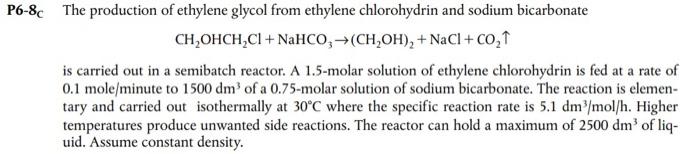
2. Consider the semibatch reactor problem from the last homework (Problem 6.8 ) a. Solve part a of the third problem from the homework due March 10 with polymath b. Solve the problem from part a of this problem without ignoring changes in volume c. Solve problem 6.8a d. Compare your answers to parts a-c of this question and explain the differences Semibatch Reactor a. Using the information provided in problem 6.8, solve for the concentration of ethylene chlorohydrin as a function of time by hand assuming that both reactants have an initial concentration of 1.5 molar and there is 1250dm3 of each in a batch reactor. Ignore changes in volume. (Hint: see chapter 7 and/or powerpoint slides for the reactor lab if you need help with the integration) CH2OHCH2Cl+NaHCO3(CH2OH)2+NaCl+CO2 is carried out in a semibatch reactor. A 1.5-molar solution of ethylene chlorohydrin is fed at a rate of 0.1 mole/minute to 1500dm3 of a 0.75 -molar solution of sodium bicarbonate. The reaction is elementary and carried out isothermally at 30C where the specific reaction rate is 5.1dm3/mol/h. Higher temperatures produce unwanted side reactions. The reactor can hold a maximum of 2500dm3 of liquid. Assume constant density
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


