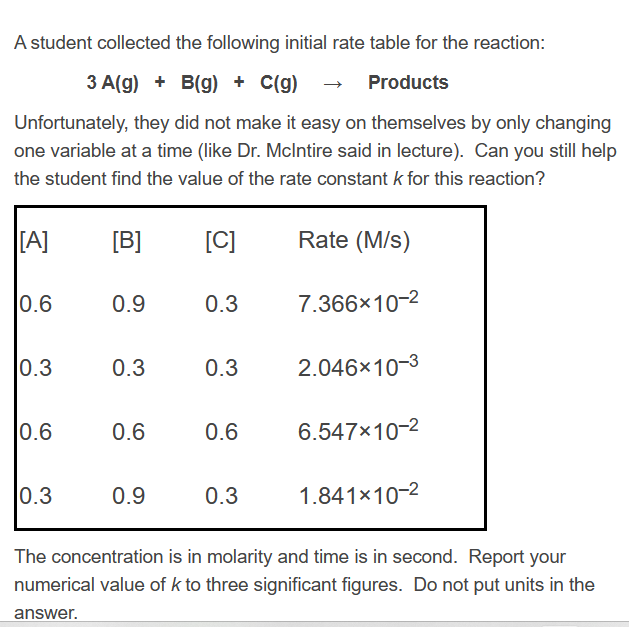
Question: Solve: A student collected the following initial rate table for the reaction: 3A(g)+B(g)+C(g)Products Unfortunately, they did not make it easy on themselves by only changing
 Solve:
Solve:
A student collected the following initial rate table for the reaction: 3A(g)+B(g)+C(g)Products Unfortunately, they did not make it easy on themselves by only changing one variable at a time (like Dr. Mclntire said in lecture). Can you still help the student find the value of the rate constant k for this reaction? The concentration is in molarity and time is in second. Report your numerical value of k to three significant figures. Do not put units in the
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


