Question: solve a-d and explain the most basic you can pls Concentration vs. Time. Determining the Order or a Reaction. (a) Determine whether this hydrogen peroxide
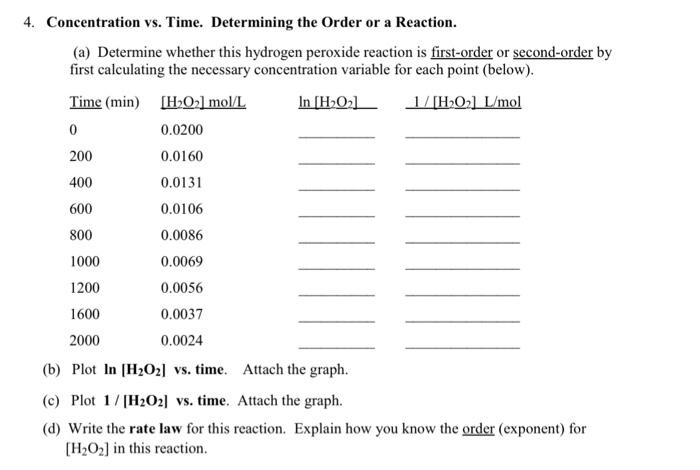
Concentration vs. Time. Determining the Order or a Reaction. (a) Determine whether this hydrogen peroxide reaction is first-order or second-order by first calculating the necessary concentration variable for each point (below). (b) Plot ln[H2O2] vs. time. Attach the graph. (c) Plot 1/[H2O2] vs. time. Attach the graph. (d) Write the rate law for this reaction. Explain how you know the order (exponent) for [H2O2] in this reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


