Question: solve and explain as well Consider the rigid system below consisting of 2kg of superheated water vapor at given initial temperature and specific volume: The
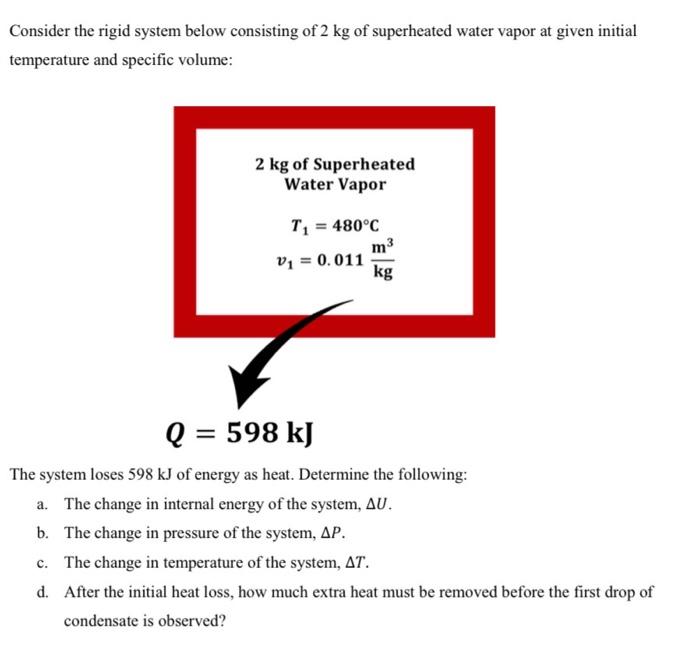
Consider the rigid system below consisting of 2kg of superheated water vapor at given initial temperature and specific volume: The system loses 598kJ of energy as heat. Determine the following: a. The change in internal energy of the system, U. b. The change in pressure of the system, P. c. The change in temperature of the system, T. d. After the initial heat loss, how much extra heat must be removed before the first drop of condensate is observed
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


