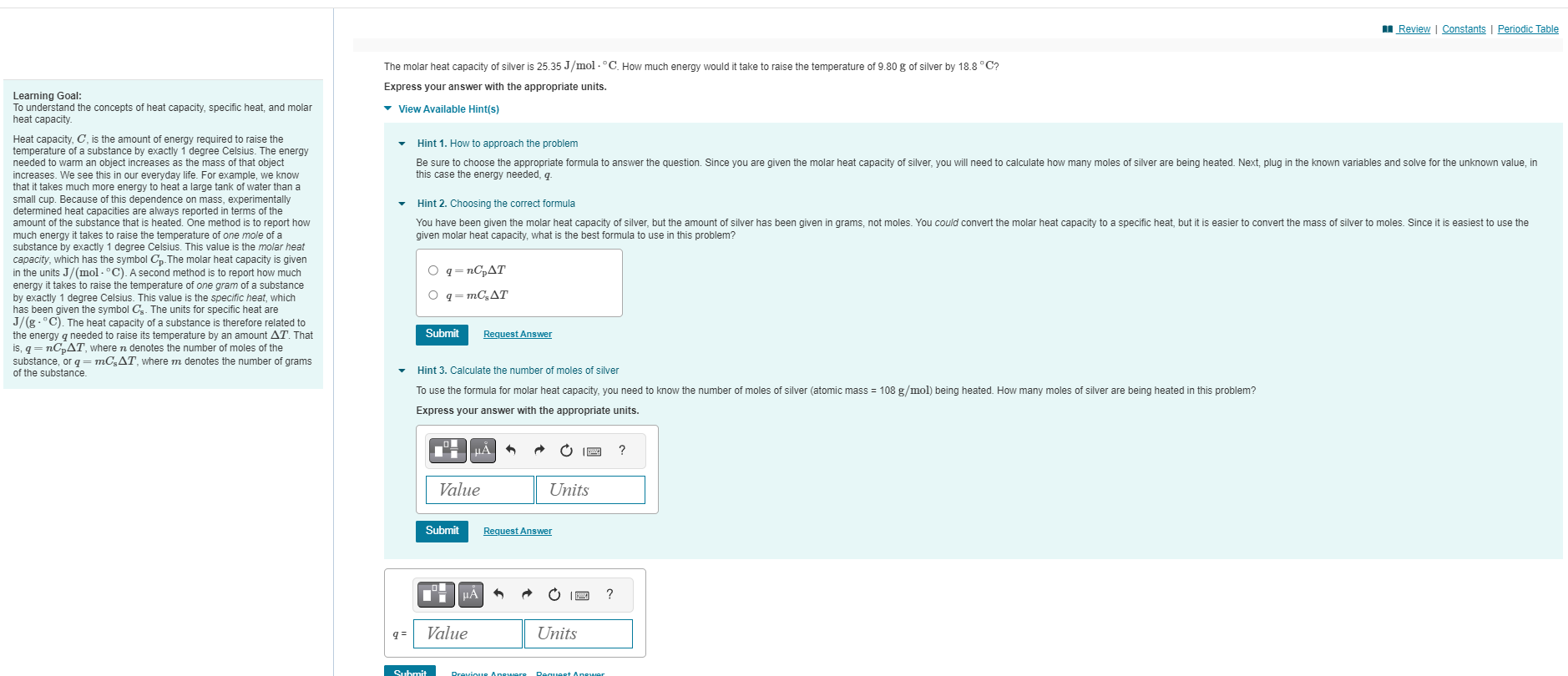
Question: solve and read through the learning goal Review | Constants | Periodic Table The molar heat capacity of silver is 25.35 J/mol . C. How
solve and read through the learning goal
Review | Constants | Periodic Table The molar heat capacity of silver is 25.35 J/mol . C. How much energy would it take to raise the temperature of 9.80 g of silver by 18.8 "C? Express your answer with the appropriate units. Learning Goal: To understand the concepts of heat capacity, specific heat, and molar View Available Hint(s) heat capacity. Heat capacity, C, is the amount of energy required to raise the Hint 1. How to approach the problem temperature of a substance by exactly 1 degree Celsius. The energy needed to warm an object increases as the mass of that object Be sure to choose the appropriate formula to answer the question. Since you are given the molar heat capacity of silver, you will need to calculate how many moles of silver are being heated. Next, plug in the known variables and solve for the unknown value, in increases. We see this in our everyday life. For example, we know this case the energy needed, q. that it takes much more energy to heat a large tank of water than a small cup. Because of this dependence on mass, experimentally determined heat capacities are always reported in terms of the Hint 2. Choosing the correct formula amount of the substance that is heated. One method is to report how You have been given the molar heat capacity of silver, but the amount of silver has been given in grams, not moles. You could convert the molar heat capacity to a specific heat, but it is easier to convert the mass of silver to moles. Since it is easiest to use the much energy it takes to raise the temperature of one mole of a given molar heat capacity, what is the best formula to use in this problem? substance by exactly 1 degree Celsius. This value is the molar heat capacity, which has the symbol Cp- The molar heat capacity is given in the units J/(mol . C). A second method is to report how much O q = nCpAT energy it takes to raise the temperature of one gram of a substance by exactly 1 degree Celsius. This value is the specific heat, which O q= mCAT has been given the symbol Cs. The units for specific heat are J/(g . "C). The heat capacity of a substance is therefore related to the energy q needed to raise its temperature by an amount AT. That Submit Request Answer is, q = nopAT, where n denotes the number of moles of the substance, or q = mCAT, where m denotes the number of grams of the substance. Hint 3. Calculate the number of moles of silver To use the formula for molar heat capacity, you need to know the number of moles of silver (atomic mass = 108 g/mol) being heated. How many moles of silver are being heated in this problem? Express your answer with the appropriate units. HA Value Units Submit Request Answer HA Value Units