Question: Solve any ONE from these two step by step answer please only hand written accepted Q2 ( Determine the equation for the fugacity coefficient of
Solve any ONE from these two step by step answer please only hand written accepted
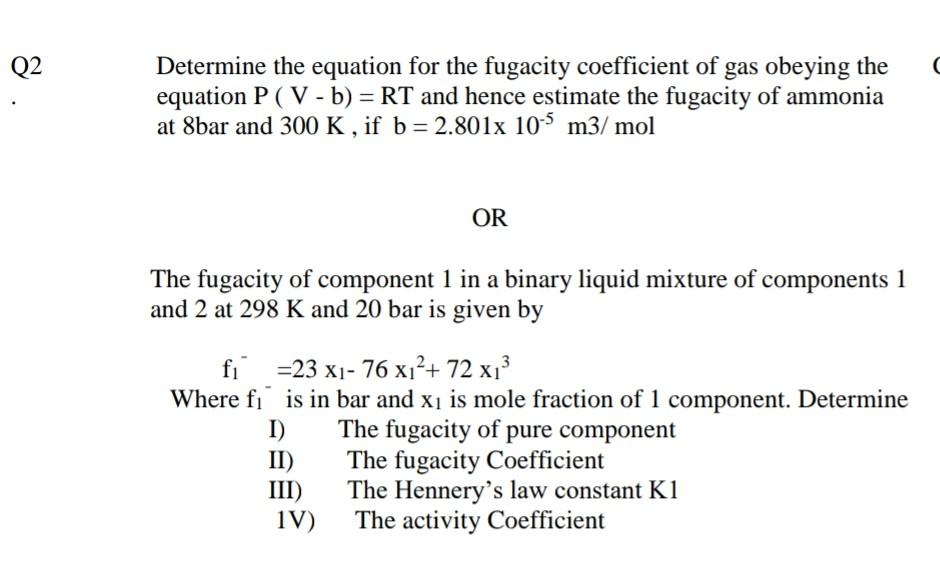
Q2 ( Determine the equation for the fugacity coefficient of gas obeying the equation P (V - b) = RT and hence estimate the fugacity of ammonia at 8bar and 300 K , if b = 2.801x 10-5 m3/mol = . OR The fugacity of component 1 in a binary liquid mixture of components 1 and 2 at 298 K and 20 bar is given by fi =23 x1- 76 x?+ 72 x} Where fi is in bar and x is mole fraction of 1 component. Determine I) The fugacity of pure component II) The fugacity Coefficient III) The Hennery's law constant K1 1V) The activity Coefficient
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


