Question: Solve any one from these two. step by step answer please only hand written accepted A gas containing 20% CO and 80% N2 is burnt
Solve any one from these two. step by step answer please only hand written accepted
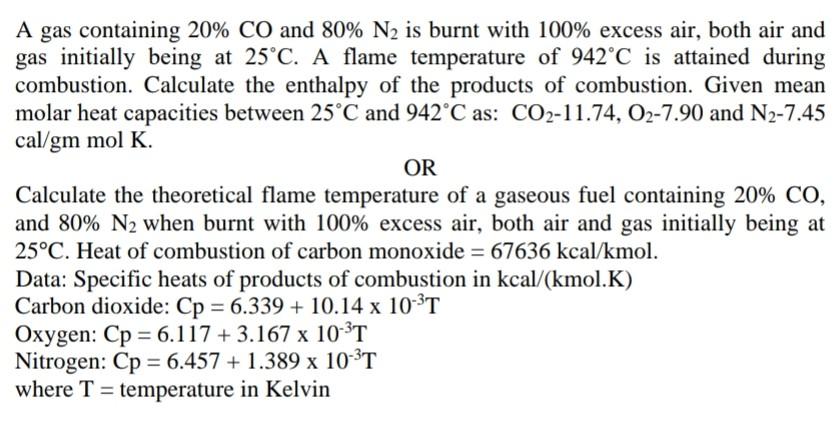
A gas containing 20% CO and 80% N2 is burnt with 100% excess air, both air and gas initially being at 25C. A flame temperature of 942C is attained during combustion. Calculate the enthalpy of the products of combustion. Given mean molar heat capacities between 25C and 942C as: CO2-11.74, 02-7.90 and N2-7.45 cal/gm mol K. OR Calculate the theoretical flame temperature of a gaseous fuel containing 20% CO, and 80% N2 when burnt with 100% excess air, both air and gas initially being at 25C. Heat of combustion of carbon monoxide = 67636 kcal/kmol. Data: Specific heats of products of combustion in kcal/(kmol.K) Carbon dioxide: Cp = 6.339 + 10.14 x 10-'T Oxygen: Cp = 6.117 + 3.167 x 10-3T Nitrogen: Cp = 6.457 + 1.389 x 10-3T where T = temperature in Kelvin
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


