Question: solve asap and correct answer is expected. B Calculate the change in entropy that occurs when 18.02 g of ice at -10.0C is placed in
solve asap and correct answer is expected. 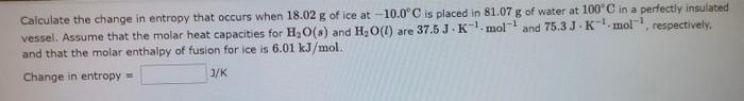
B Calculate the change in entropy that occurs when 18.02 g of ice at -10.0C is placed in 81.07 g of water at 100C in a perfectly insulated vessel. Assume that the molar heat capacities for H,0(s) and H2O(l) are 37.5J K.mol-1 and 75.3J.K-mol, respectively. and that the molar enthalpy of fusion for ice is 6.01 kJ/mol. Change in entropy 3/K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


