Question: solve ASAP and give Correct answer The table below shows data for molecule X, which has a molecular weight of 58.9 g/mol. Use the data
solve ASAP and give Correct answer
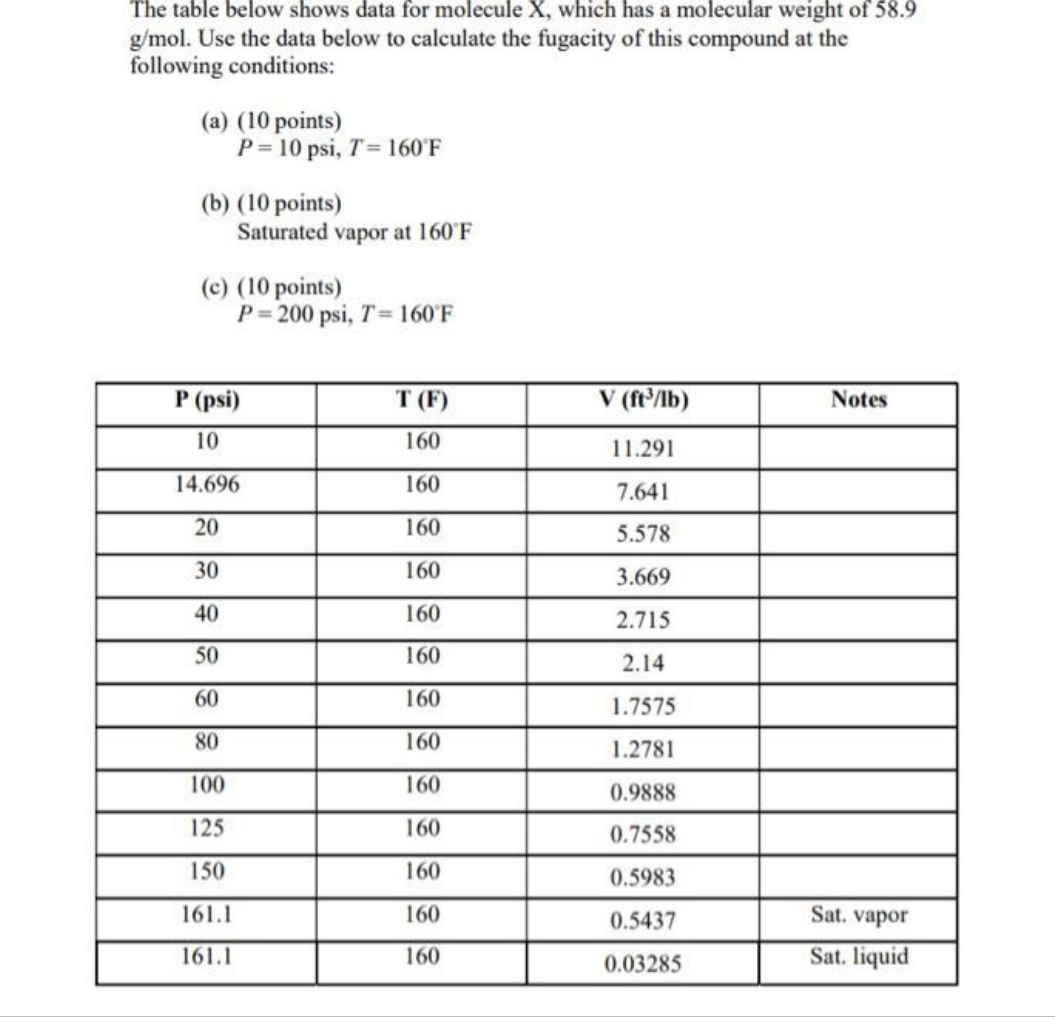
The table below shows data for molecule X, which has a molecular weight of 58.9 g/mol. Use the data below to calculate the fugacity of this compound at the following conditions: (a) (10 points) P = 10 psi, T= 160 F (b) (10 points) Saturated vapor at 160"F (c) (10 points) P = 200 psi, T= 160'F P (psi) T (F) V (ft3/1b) Notes 10 160 11.291 14.696 160 7.641 20 160 5.578 30 160 3.669 40 160 2.715 50 160 2.14 60 160 1.7575 80 160 1.2781 100 160 0.9888 125 160 0.7558 150 160 0.5983 161.1 160 0.5437 Sat. vapor 161.1 160 0.03285 Sat. liquid
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


