Question: The table below shows data for molecule X, which has a molecular weight of 58.9 g/mol. Use the data below to calculate the fugacity of
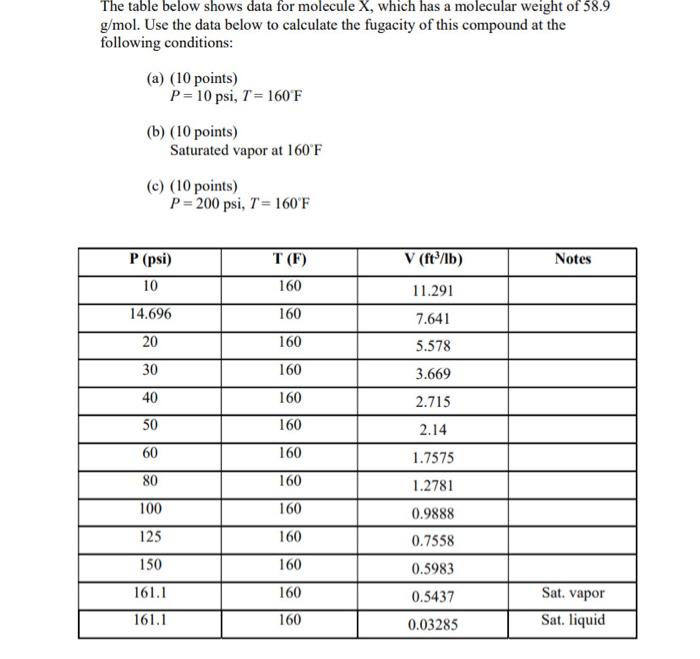
The table below shows data for molecule X, which has a molecular weight of 58.9 g/mol. Use the data below to calculate the fugacity of this compound at the following conditions: (a) (10 points) P=10psi,T=160F (b) (10 points) Saturated vapor at 160F (c) (10 points) P=200psi,T=160F
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


