Question: solve both pls Required information NOTE: This is a multt-part question. Once an answer is submitted, you will be unable to return to this part.
 solve both pls
solve both pls
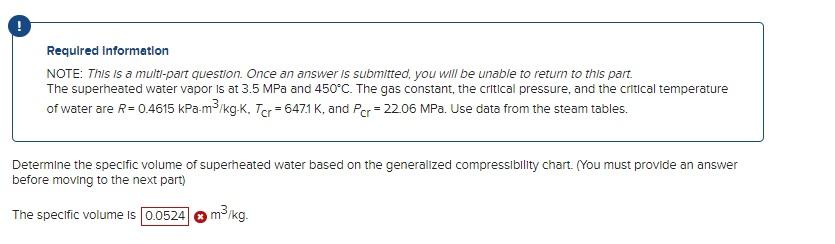
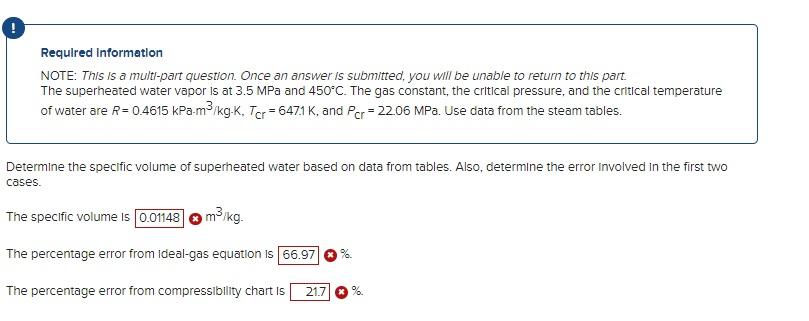
Required information NOTE: This is a multt-part question. Once an answer is submitted, you will be unable to return to this part. The superheated water vapor is at 3.5MPa and 450C. The gas constant, the critical pressure, and the critical temperature of water are R=0.4615kPam3/kgK,Tcr=647.1K, and PCr=22.06MPa. Use data from the steam tables. DetermIne the specific volume of superheated water based on the generalized compressibility chart. (You must provide an answer before moving to the next part) The specific volume is m3/kg Requlred Information NOTE: This is a mult-part question. Once an answer is submitted, you will be unable to return to this part. The superheated water vapor is at 3.5MPa and 450C. The gas constant, the crltical pressure, and the critical temperature of water are R=0.4615kPam3/kgK,TCr=647.1K, and PCr=22.06MPa. Use data from the steam tables. Determine the specific volume of superheated water based on data from tables. Also, determine the error involved in the first two cases. The specific volume is m3/kg. The percentage error from ideal-gas equation is The percentage error from compresslbility chart is . %
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


