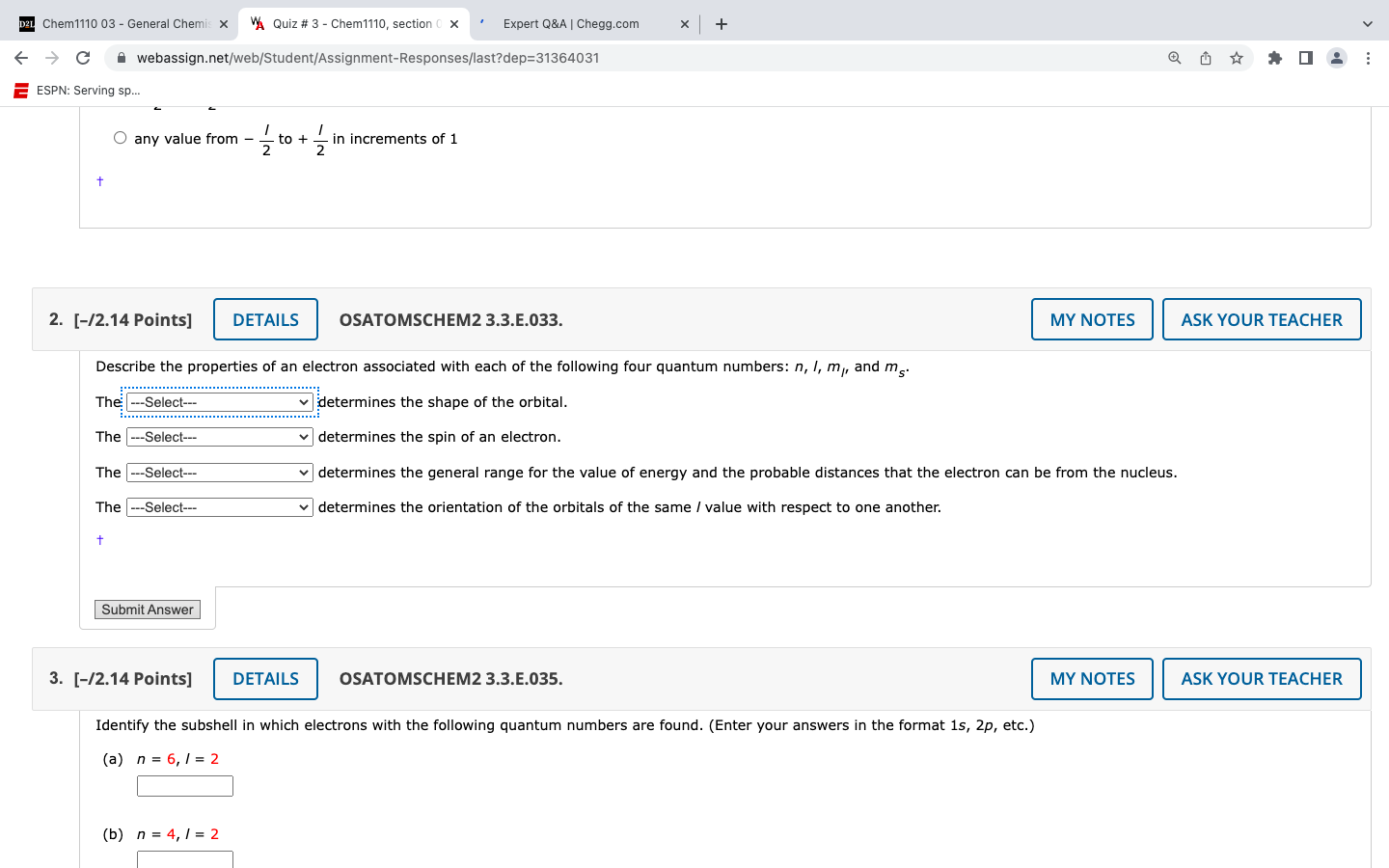
Question: Solve for question 2. any value from 21 to +21 in increments of 1 [12.14 Points ] OSATOMSCHEM2 3.3.E.033. Describe the properties of an electron
 Solve for question 2.
Solve for question 2.
any value from 21 to +21 in increments of 1 [12.14 Points ] OSATOMSCHEM2 3.3.E.033. Describe the properties of an electron associated with each of the following four quantum numbers: n,l,ml, and ms. The determines the shape of the orbital. The determines the spin of an electron. The determines the general range for the value of energy and the probable distances that the electron can be from the nucleus. The determines the orientation of the orbitals of the same I value with respect to one another. [12.14 Points ] OSATOMSCHEM2 3.3.E.035. Identify the subshell in which electrons with the following quantum numbers are found. (Enter your answers in the format 1s, 2p, etc.) (a) n=6,I=2 (b) n=4,I=2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


