Question: Solve for what is being asked and DO NOT COPY the other solutions here in chegg because they are all wrong! (15 points) We are
Solve for what is being asked and DO NOT COPY the other solutions here in chegg because they are all wrong!
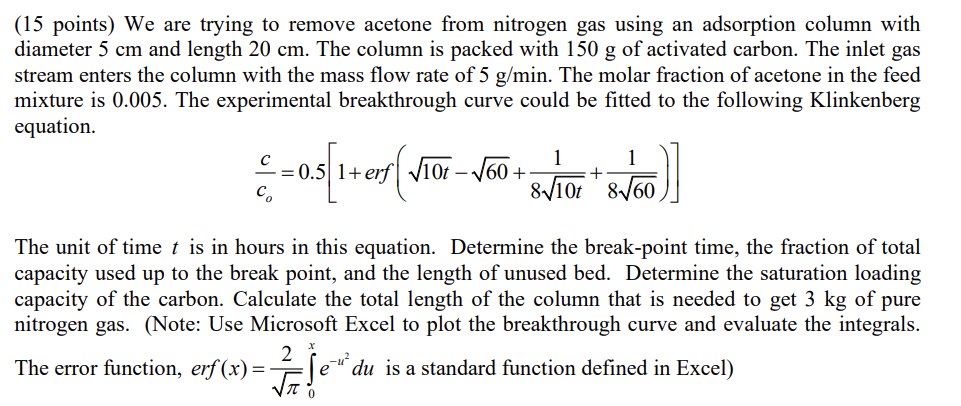
(15 points) We are trying to remove acetone from nitrogen gas using an adsorption column with diameter 5cm and length 20cm. The column is packed with 150g of activated carbon. The inlet gas stream enters the column with the mass flow rate of 5g/min. The molar fraction of acetone in the feed mixture is 0.005 . The experimental breakthrough curve could be fitted to the following Klinkenberg equation. coc=0.5[1+erf(10t60+810t1+8601)] The unit of time t is in hours in this equation. Determine the break-point time, the fraction of total capacity used up to the break point, and the length of unused bed. Determine the saturation loading capacity of the carbon. Calculate the total length of the column that is needed to get 3kg of pure nitrogen gas. (Note: Use Microsoft Excel to plot the breakthrough curve and evaluate the integrals. The error function, erf(x)=20xeu2du is a standard function defined in Excel)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


