Question: solve in 60 mins thanks b. (25 points) Assume a stream of carbon dioxide at 1 atm and 100C blows across a 1-mm naphthalene sphere
solve in 60 mins thanks 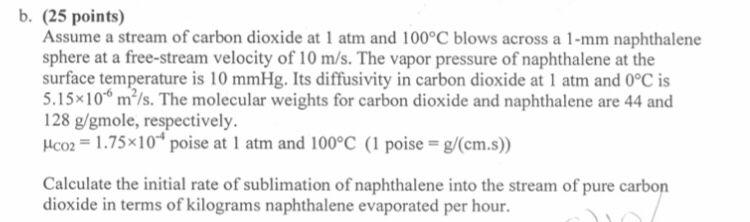
b. (25 points) Assume a stream of carbon dioxide at 1 atm and 100C blows across a 1-mm naphthalene sphere at a free-stream velocity of 10 m/s. The vapor pressure of naphthalene at the surface temperature is 10 mmHg. Its diffusivity in carbon dioxide at 1 atm and 0C is 5.15x10 m/s. The molecular weights for carbon dioxide and naphthalene are 44 and 128 g/gmole, respectively. Mco2 = 1.75*10* poise at 1 atm and 100C (1 poise = g/(cm.s)) Calculate the initial rate of sublimation of naphthalene into the stream of pure carbon dioxide in terms of kilograms naphthalene evaporated per hour
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


