Question: solve in python please The vdW equation is a cubic equation of state and similar to the ideal gas law. It requires two state variables
solve in python please
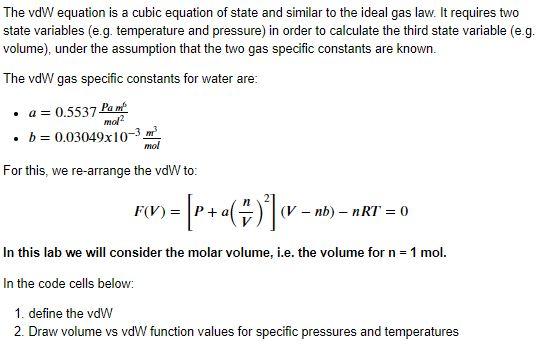
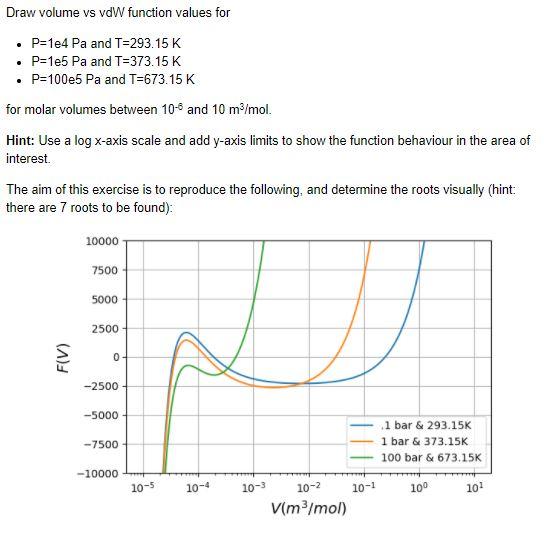
The vdW equation is a cubic equation of state and similar to the ideal gas law. It requires two state variables (e.g. temperature and pressure) in order to calculate the third state variable (e.g. volume), under the assumption that the two gas specific constants are known. The vdW gas specific constants for water are: . a = 0.5537 Pan mol . b=0.03049x10-3 mol For this, we re-arrange the vdW to: F(V) = (V - nb) - nRT = 0 In this lab we will consider the molar volume, i.e. the volume for n = 1 mol. In the code cells below: 1. define the vdW 2. Draw volume vs vd function values for specific pressures and temperatures Draw volume vs vd function values for P=1e4 Pa and T=293.15 K P=1e5 Pa and T=373.15 K P=100e5 Pa and T=673.15 K for molar volumes between 10- and 10 m3/mol. Hint: Use a log x-axis scale and add y-axis limits to show the function behaviour in the area of interest The aim of this exercise is to reproduce the following, and determine the roots visually (hint: there are 7 roots to be found): 10000 7500 5000 2500 F(V) 0 -2500 -5000 -7500 1 bar & 293.15K 1 bar & 373.15K 100 bar & 673.15K -10000 10-5 10-4 10-3 100 101 10-2 10-1 V(m2/mol)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


