Question: Solve last part only related to the graph. Equal 1 cm2 areas of metals P and Q are immersed together in sulfuric acid of unit
Solve last part only related to the graph.
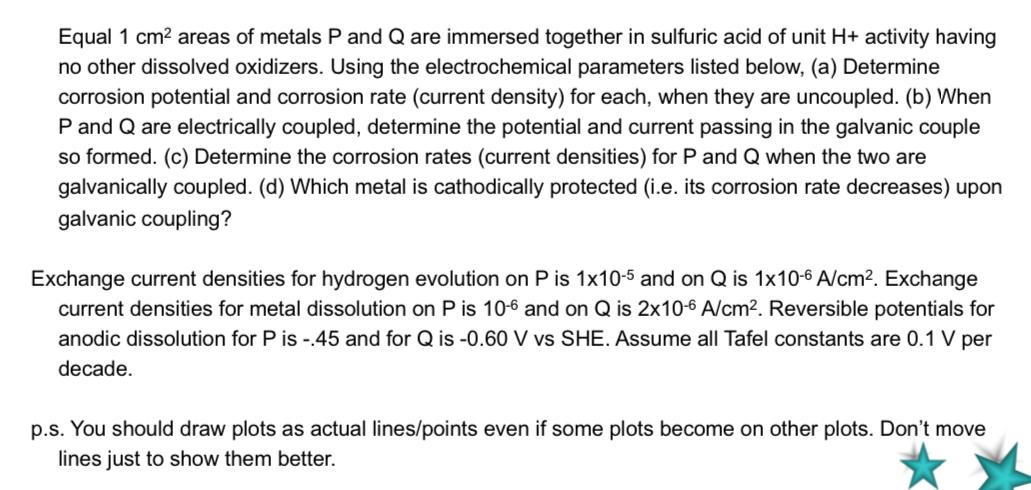
Equal 1 cm2 areas of metals P and Q are immersed together in sulfuric acid of unit H+ activity having no other dissolved oxidizers. Using the electrochemical parameters listed below, (a) Determine corrosion potential and corrosion rate (current density) for each, when they are uncoupled. (b) When P and Q are electrically coupled, determine the potential and current passing in the galvanic couple so formed. (c) Determine the corrosion rates (current densities) for P and Q when the two are galvanically coupled. (d) Which metal is cathodically protected (i.e. its corrosion rate decreases) upon galvanic coupling? Exchange current densities for hydrogen evolution on P is 1x10-5 and on Q is 1x10-6 A/cm2. Exchange current densities for metal dissolution on P is 10-6 and on Q is 2x10-6 A/cm2. Reversible potentials for anodic dissolution for Pis - 45 and for Q is -0.60 V vs SHE. Assume all Tafel constants are 0.1 V per decade. p.s. You should draw plots as actual lines/points even if some plots become on other plots. Don't move lines just to show them better
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


