Question: SOLVE ME! 1. Determine S for the reaction: SO3{g) + H20(1) H2SO4(1) Given: S(J/K.mol): 156.9 256.2 69.9 2. Calcuiate S for the reaction SO2(s)
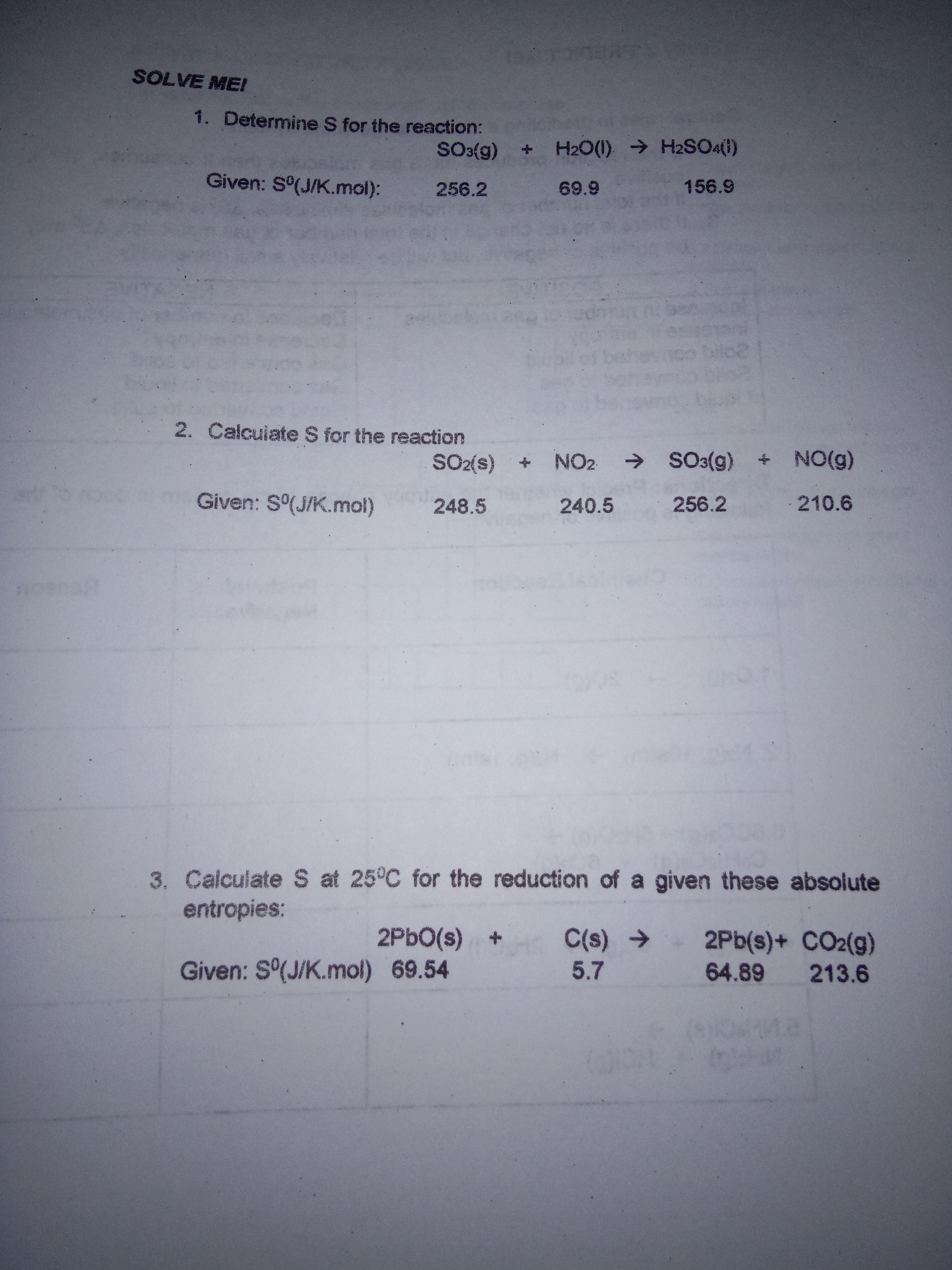
SOLVE ME! 1. Determine S for the reaction: SO3{g) + H20(1) H2SO4(1) Given: S(J/K.mol): 156.9 256.2 69.9 2. Calcuiate S for the reaction SO2(s) NO2 SO3(g) + NO(g) Given: S(J/K.mol) 248.5 240.5 256.2 210.6 3. Calculate S at 25C for the reduction of a given these absolute entropies: 2PBO(s) + Given: S(J/K.mol) 69.54 C(s) 2Pb(s)+ CO2(g) 5.7 64.89 213.6
Step by Step Solution
★★★★★
3.45 Rating (155 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


