Use Hesss law to determine r H for the reaction C 3 H 4 (g) +
Question:
Use Hess’s law to determine ΔrH° for the reaction C3H4(g) + 2 H2(g) → C3H8(g), given that
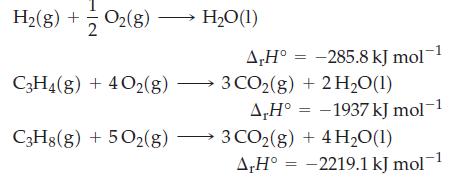
Transcribed Image Text:
H₂(g) + O2(8) O₂(g) → C3H4(g) + 4O2(g) C3H8(g) + 5O2(g) H₂O(1) A,H° -285.8 kJ mol-1 3 CO2(g) + 2H₂O(1) A,H° -1937 kJ mol-1 3 CO2(g) + 4H₂O(1) A,H° -2219.1 kJ mol-1 = =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Using Hesss law we can determine the enthalpy of reaction for ...View the full answer

Answered By

Vikash Gupta
I am graduated in Physics in 2018, from KIRORIMAL COLLEGE, University of Delhi. Now I am persuing Master's degree in physics. I like to do physics problems. I have experience of 1 year in tutoring. I think Physics is the only subject where you understand things,how they are happening . In physics you learn Maths and apply it. So I would like to join your platform to solve many Physics problems.
5.00+
5+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The gas-phase reaction between Br2 and H2 to form HBr is assumed to proceed by the following mechanism: a. Under what conditions does the rate law have the form rate = k[Br2]? b. Under what...
-
The equilibrium constant for the reaction H2 + at 1 atm and 1500C is given to be K. Of the reactions given below, all at 1500C, the reaction that has a different equilibrium constant is (a) H2 + 12O2...
-
The decomposition of NH3 to N2 and H2 was studied on two surfaces: Without a catalyst, the activation energy is 335 kJ/ mol. a. Which surface is the better heterogeneous catalyst for the...
-
A heat engine operates between two reservoirs at 800 and 20C. One-half of the work output of the heat engine is used to drive a Carnot heat pump that removes heat from the cold surroundings at 2C and...
-
Given the following selected account balances of Spalding Company, prepare its manufacturing statement for the year ended on December 31, 2009. Include a listing of the individual overhead account...
-
Based on the Beneish model results for 2017, which company has the highest probability of being an earnings manipulator? A . BIG Industrial B . Construction Supply C . Dynamic Production
-
Fun-Tastic Shows, Inc., is a company that hosts carnivals and similar events. Susan Swartwood, Crystal Groth, and a minor (named in the case as M.G.S.) attended Fun-Tastics Rhododendron Festival in...
-
For the past several years, Jeff Horton has operated a part-time consulting business from his home. As of April 1, 2014, Jeff decided to move to rented quarters and to operate the business, which was...
-
Why won't a relational model work with Big Data?
-
Determine the theoretical and actual material required to produce the part, shown in figure. Raw stock is supplied in 0.875 in. diameter. A lathe cutoff tool width is 0 125 in. A 0.015 in. stock...
-
Given the following information: 3 N(g) + -H(g) NH3(g) A,Hi 3 NO(g) + HO(1) A,H2 HO(1) A,H3 Determine A,H for the following reaction, expressed in terms of A,Hi, A,H2, and A,H. N(g) + O(g) 2 NO(g)...
-
Use Hesss law to determine r H for the reaction 1 CO(g) + O2(g) CO(g), given that C(graphite) + +10(8) C(graphite) + O(g) CO(g) A,H -110.54 kJ mol-1 CO(g) A,H = -393.51 kJ mol-1 =
-
Steam exiting the turbine of a steam power plant at 100°F is to be condensed in a large condenser by cooling water flowing through copper pipes (k = 223 Btu/h·ft·°F) of inner...
-
NewTech purchases computer equipment for $264,000 to use in operating activities for the next four years. It estimates the equipments salvage value at $28,000. rev: 07_27_2017_QC_CS-94103 Exercise...
-
Assuming riskless arbitrage opportunities do not exist, one cannot expect to have a return greater than the risk-free rate without taking on some risk. In order to obtain greater returns on...
-
Suppose your current monthly net salary is 1000 euros, presumably it should increase every year by 6.0% until your retirement (you are planning to retire exactly in 25 years from now, your planned...
-
Misty's gross pay is $770 per week. Misty has provided her TFN and claims the tax-free threshold. This week Misty goes on leave and is to be paid 6 weeks leave in advance. Misty is not eligible for...
-
what is the yield to maturity for a bond that matures in 15 years, is callable in 6 years ,has a coupon rate of 4.5% and a price of $995 and a call premium of $45?
-
Blue Jeans Shop had net retail sales of $390,000 during the current year. The following additional information was obtained from the companys accounting records: Using the retail method, estimate the...
-
Construct a 4 x 25 design confounded in two blocks of 16 observations each. Outline the analysis of variance for this design.
-
Simmons Corporation owns stock of Armstrong, Inc prior to 2010 the investment was accounted for using the equity method. In early 2010, Simmons sold part of its investment in Armstrong, and began...
-
Oliver Corporation has owned stock of Conrad Corporation since 2007. At December 31, 2010, its balances related to this investment were: Available-for-Sale Securities $185,000 Securities Fair Value...
-
Change in PrincipleLong-Term Contracts Cherokee Construction Company changed from the completed-contract to the percentage-of-completion method of accounting for long-term construction contracts...
-
Subject Label 1: yellow Label 2: green red blue Write a complete webpage using HTML and CSS that 1) Make a webpage with two textboxes at the top and a 3 3 table at the bottom, as shown in above...
-
41. A rock is thrown upward, and its height in yards after t seconds is given by h(t)=-16t2 +27t + 2 What is the maximum height it reaches, and when does it hit the ground?
-
What is Criminal Law,how does steal something from work can be related to Criminal Law ?

Study smarter with the SolutionInn App


