Question: SOLVE PLEASE From the Hc and Hf tables, I've calculated that the standard heat of my gasphase reaction at 25C is as follows: A+B2RHr,298K=50000J At
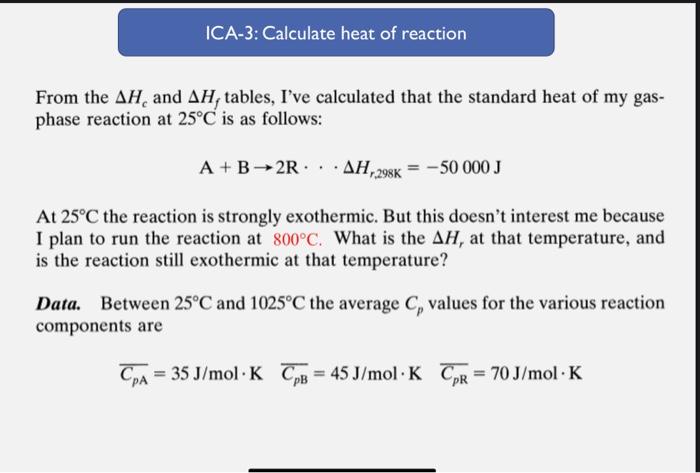
From the Hc and Hf tables, I've calculated that the standard heat of my gasphase reaction at 25C is as follows: A+B2RHr,298K=50000J At 25C the reaction is strongly exothermic. But this doesn't interest me because I plan to run the reaction at 800C. What is the Hr at that temperature, and is the reaction still exothermic at that temperature? Data. Between 25C and 1025C the average Cp values for the various reaction components are CpA=35J/molKCpB=45J/molKCpR=70J/molK
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


