Question: solve please Q1) At a particular location in a countercurrent stripper for the removal of solute A from a liquid stream, the mole fraction of
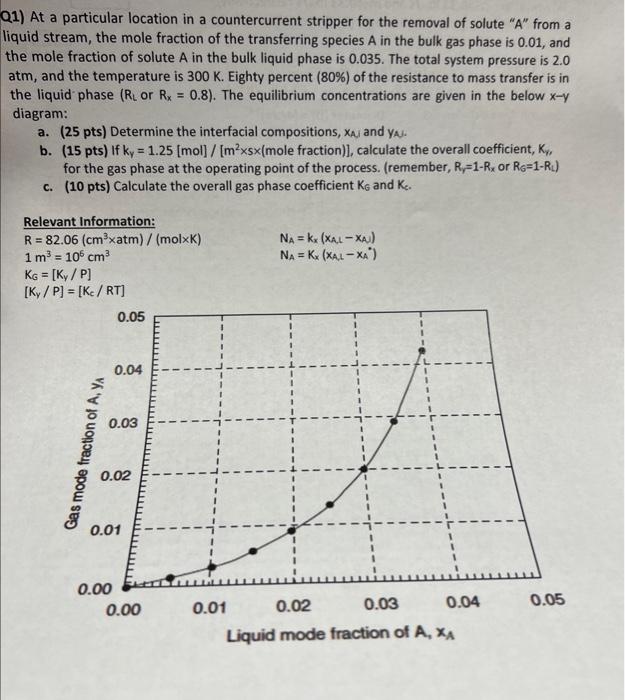
Q1) At a particular location in a countercurrent stripper for the removal of solute "A" from a liquid stream, the mole fraction of the transferring species A in the bulk gas phase is 0.01 , and the mole fraction of solute A in the bulk liquid phase is 0.035 . The total system pressure is 2.0 atm, and the temperature is 300K. Eighty percent (80%) of the resistance to mass transfer is in the liquid phase ( RL or Rx=0.8 ). The equilibrium concentrations are given in the below xy diagram: a. (25 pts) Determine the interfacial compositions, xA and yA. b. (15 pts) If ky=1.25[mol]/[m25 (mole fraction)], calculate the overall coefficient, Kr, for the gas phase at the operating point of the process. (remember, RY=1Rx or R6=1RL ) c. (10 pts) Calculate the overall gas phase coefficient KG and Kc. Relevant Information: R=82.06(cm3atm)/(molxK)1m3=106cm3KG=[Ky/P][Ky/P]=[Kc/RT]NA=kx(xA,1xA)NA=Kx(xALxA)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


