Question: Solve quickly for a thumbs up Question 2 10 pts Methane can be converted into formaldehyde by the following reaction: CH4 + O2 HCHO +
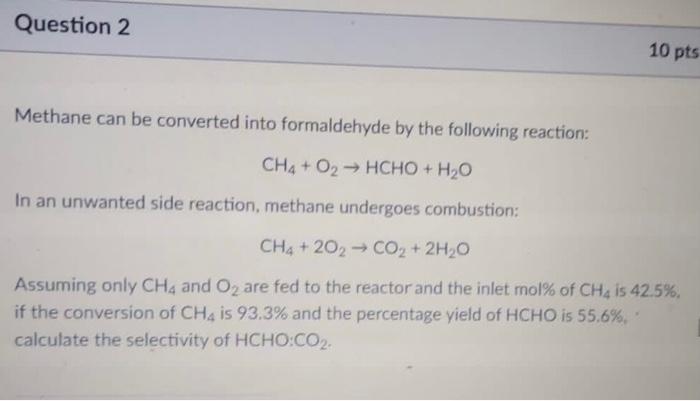
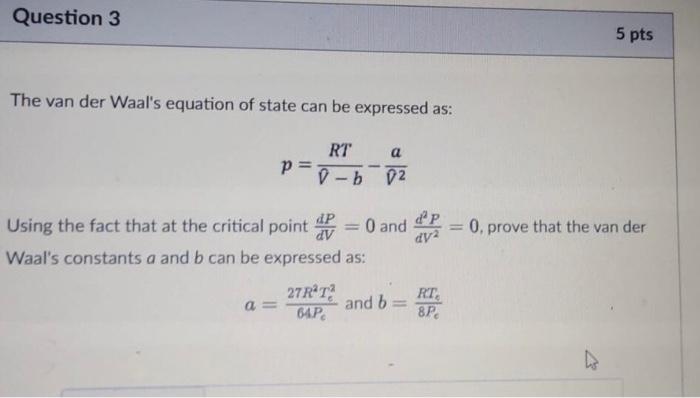
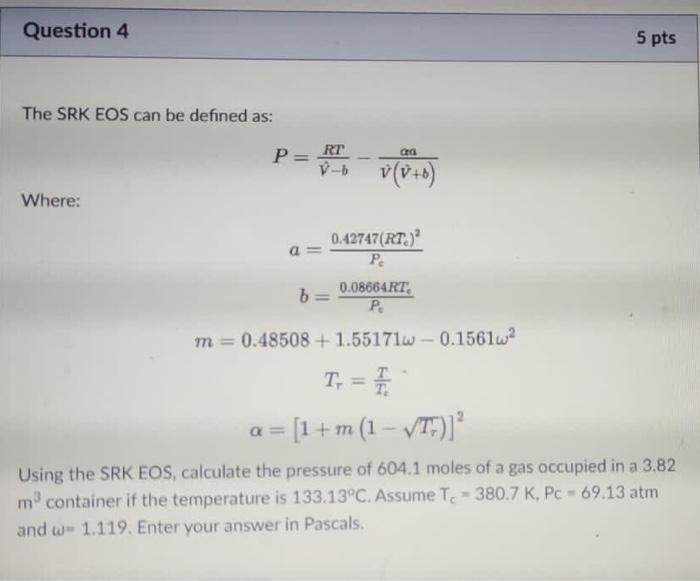
Question 2 10 pts Methane can be converted into formaldehyde by the following reaction: CH4 + O2 HCHO + H2O In an unwanted side reaction, methane undergoes combustion: CH4 + 202 CO2 + 2H2O Assuming only CH4 and O2 are fed to the reactor and the inlet mol% of CH4 is 42.5%, if the conversion of CH4 is 93.3% and the percentage yield of HCHO is 55.6%, calculate the selectivity of HCHO:CO2- Question 3 5 pts The van der Waal's equation of state can be expressed as: RT a D-b 02 - P = 0. prove that the van der Using the fact that at the critical point out = 0 and dap dy2 Waal's constants a and b can be expressed as: 27RT RT, and b 64P 8P = Question 4 5 pts The SRK EOS can be defined as: a P= RT 7- v(v+6) Where: a 0.42747(RT) PC b 0.08664RT. . m = 0.48508 +1.55171w -0.1561w2 T: = T. = [1 + m (1 - VT;)] Using the SRK EOS, calculate the pressure of 604.1 moles of a gas occupied in a 3.82 mcontainer if the temperature is 133.13C. Assume Tc - 380.7 K, Pc - 69.13 atm and w- 1.119. Enter your answer in Pascals
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


