Question: Solve quicky for a thumbs up Question 1 10 pts Methane can be converted into formaldehyde by the following reaction: CH4 + O2 HCHO +
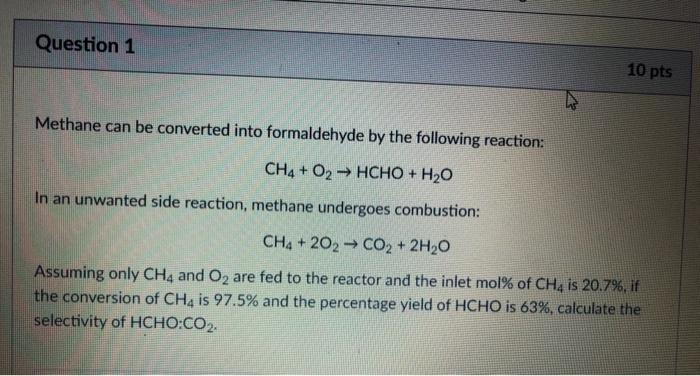

Question 1 10 pts Methane can be converted into formaldehyde by the following reaction: CH4 + O2 HCHO + H20 In an unwanted side reaction, methane undergoes combustion: CH4 + 202 CO2 + 2H2O Assuming only CH4 and O2 are fed to the reactor and the inlet mol% of CH4 is 20.7%, if the conversion of CH4 is 97.5% and the percentage yield of HCHO is 63%, calculate the selectivity of HCHO:CO2. Question 2 10 pts A fuel that is comprised of 68.7 mol% atomic C, 12.2 mol% atomic H, 1.8 mol% atomic S and the remainder incombustible inert solids is fed to a combustion chamber where it is burned with 11% excess air. For the excess air calculation, it is assumed that the C is converted to CO2, H is converted to H2O and S is converted to SO2. In reality, it was found that 14.1% of the inlet C underwent incomplete combustion to form CO, while all the remaining atoms underwent complete combustion. Calculate the mol fraction of CO in the output gas stream (enter your answer to 4 decimal places)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


