Question: solve the 5 math questions please as soon as possible Version 202203A 1. A 2.3 m beam has a mass of 110.5 g. At this
solve the 5 math questions please as soon as possible




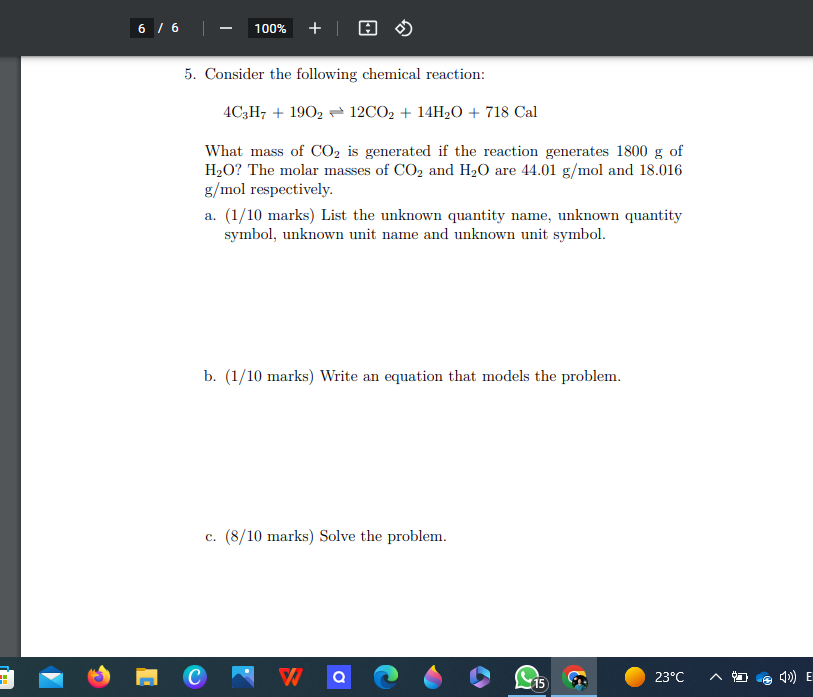
Version 202203A 1. A 2.3 m beam has a mass of 110.5 g. At this rate, what is the mass of 3.3 m of this beam? a. (1/10 marks) List the unknown quantity name, unknown quantity symbol, unknown unit name and unknown unit symbol. b. (1/10 marks) Write an equation that models the problem. c. (8/10 marks) Solve the problem. Q 10:0 23 C A DJ @ ()) ENG 2023MATH1142 Problem-Solving Skills (PSS) Test 3 3 2. The reaction generated 1800 Cal of heat. Convert this to k.J. 1 k.J = 239 cal 1 Cal = 1000 cal a. (1/10 marks) List the unknown quantity name, unknown quantity symbol, unknown unit name and unknown unit symbol. b. (1/10 marks) Write an equation that models the problem. c. (8/10 marks) Solve the problem. M C V7 Q 23 CVersion 202203A 3. 315 g of a solution has a volume of 4.2 L. 5 L of this solution sells for $9.29. At these rates, what mass of the solution can we buy with $120? a. (1/10 marks) List the unknown quantity name, unknown quantity symbol, unknown unit name and unknown unit symbol. b. (1/10 marks) Write an equation that models the problem. c. (8/10 marks) Solve the problem. M C Q 23 C A " @ ()ENGMATHIT42 Problem-Solving Skins (POD, Test s 4. Consider the following chemical reaction: 403H7 + 1902 - 12CO2 + 14H20 + 718 Cal What mass of Oz is used if the reaction generates 4250 Cal of heat? The molar mass of O2 is 32.00 g/mol. a. (1/10 marks) List the unknown quantity name, unknown quantity symbol, unknown unit name and unknown unit symbol. b. (1/10 marks) Write an equation that models the problem. c. (8/10 marks) Solve the problem.6 / 6 100% + 5. Consider the following chemical reaction: 4C3H7 + 1902 - 12CO2 + 14H20 + 718 Cal What mass of CO2 is generated if the reaction generates 1800 g of H20? The molar masses of CO, and HO are 44.01 g/mol and 18.016 g/mol respectively. a. (1/10 marks) List the unknown quantity name, unknown quantity symbol, unknown unit name and unknown unit symbol. b. (1/10 marks) Write an equation that models the problem. c. (8/10 marks) Solve the problem. Q 23 C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


