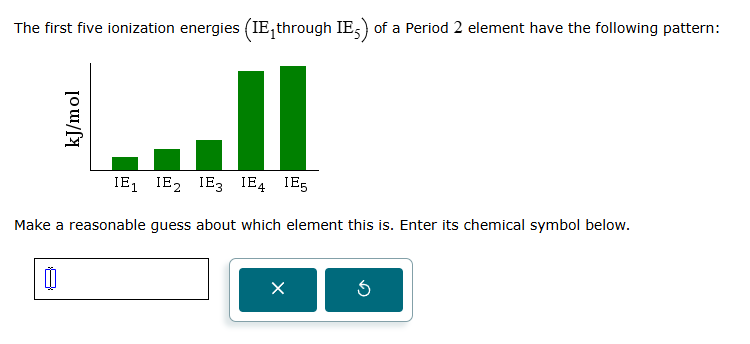
Question: solve The first five ionization energies (IE, through IE, ) of a Period 2 element have the following pattern: kJ/mol IE, IE2 IEg IE4 IES
solve
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


