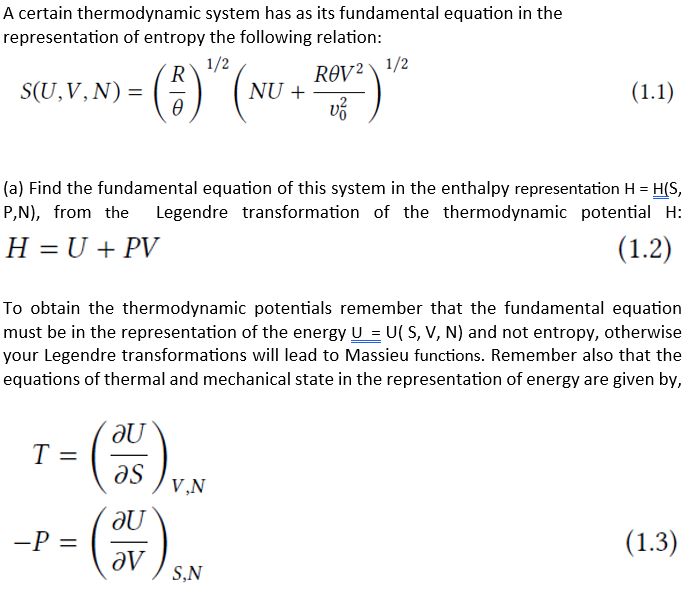
Question: Solve the problem above. Detail mathematically. A certain thermodynamic system has as its fundamental equation in the representation of entropy the following relation: S(U,V,N)=(R)1/2(NU+v02RV2)1/2 (a)

Solve the problem above. Detail mathematically.
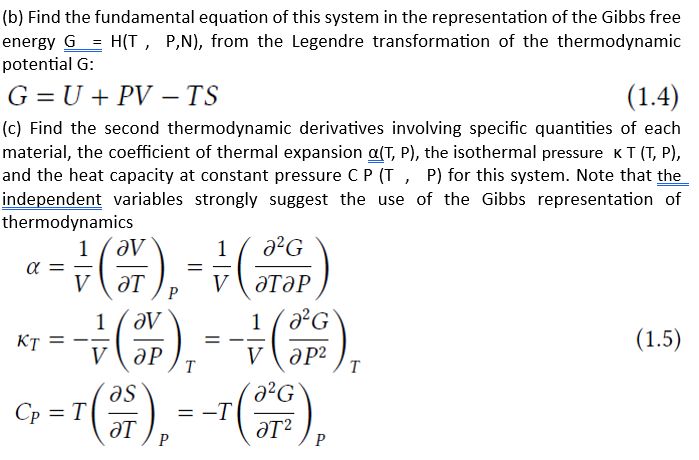
A certain thermodynamic system has as its fundamental equation in the representation of entropy the following relation: S(U,V,N)=(R)1/2(NU+v02RV2)1/2 (a) Find the fundamental equation of this system in the enthalpy representation H=H(S, P,N ), from the Legendre transformation of the thermodynamic potential H : H=U+PV To obtain the thermodynamic potentials remember that the fundamental equation must be in the representation of the energy U=U(S,V,N) and not entropy, otherwise your Legendre transformations will lead to Massieu functions. Remember also that the equations of thermal and mechanical state in the representation of energy are given by, TP=(SU)V,N=(VU)S,N (b) Find the fundamental equation of this system in the representation of the Gibbs free energy G=H(T,P,N), from the Legendre transformation of the thermodynamic potential G : G=U+PVTS (c) Find the second thermodynamic derivatives involving specific quantities of each and the heat capacity at constant pressure C P (T, P) for this system. Note that the independent variables strongly suggest the use of the Gibbs representation of thermodynamics TCP=V1(TV)P=V1(TP2G)=V1(PV)T=V1(P22G)T=T(TS)P=T(T22G)P where in equations (1.4) the second equality was obtained by substituting the equations of state in the Gibbs representation, VS=(PG)T,N=(TG)P,N
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


