Question: solve the problem step by step 1) Calculate the vapor-liquid equilibrium of the mixture (i.g., obtain the pressure and vapor composition), at the specified temperature
solve the problem step by step
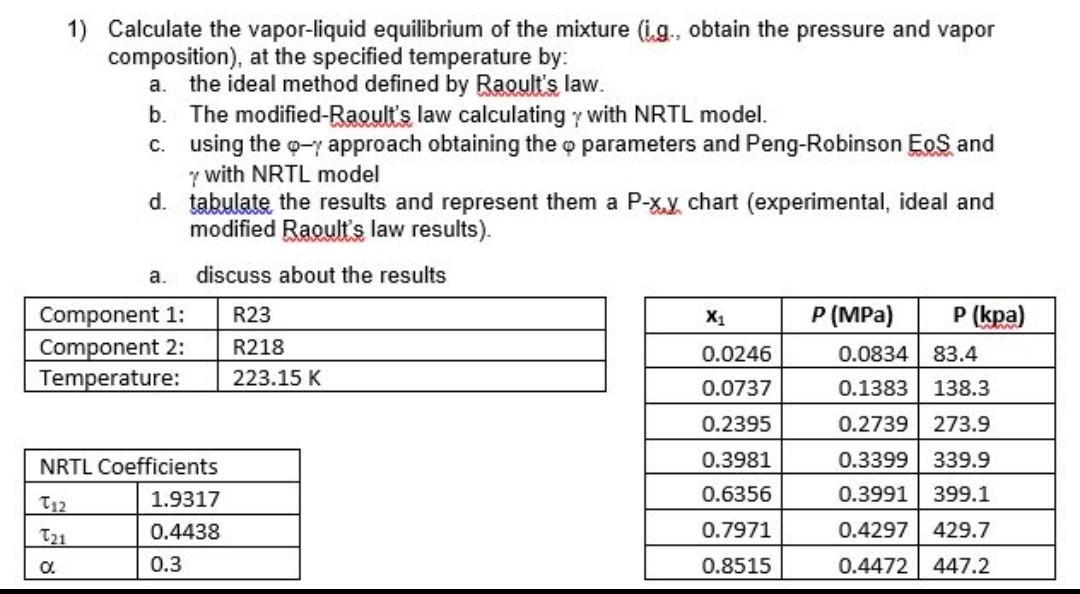
1) Calculate the vapor-liquid equilibrium of the mixture (i.g., obtain the pressure and vapor composition), at the specified temperature by: a. the ideal method defined by Raoult's law. b. The modified-Raoult's law calculating with NRTL model. c. using the approach obtaining the parameters and Peng-Robinson EoS and with NRTL model d. tabulate the results and represent them a P-xuy chart (experimental, ideal and modified Raoult's law results)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


