Question: *Solve the problem with material balances** Diethyl ether is produced by dehydration of ethanol in the presence of sulfuric acid at 140 C: 2C2H5OHC2H5OC2H5+H2O n
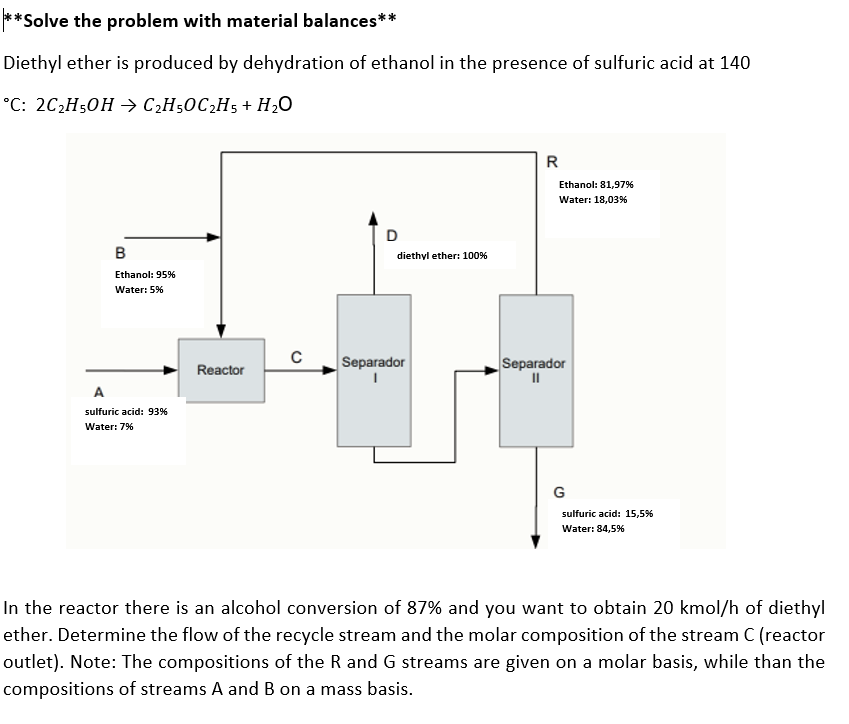
*Solve the problem with material balances** Diethyl ether is produced by dehydration of ethanol in the presence of sulfuric acid at 140 C: 2C2H5OHC2H5OC2H5+H2O n the reactor there is an alcohol conversion of 87% and you want to obtain 20kmol/h of diethyl ther. Determine the flow of the recycle stream and the molar composition of the stream C (reactor putlet). Note: The compositions of the R and G streams are given on a molar basis, while than the compositions of streams A and B on a mass basis
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


